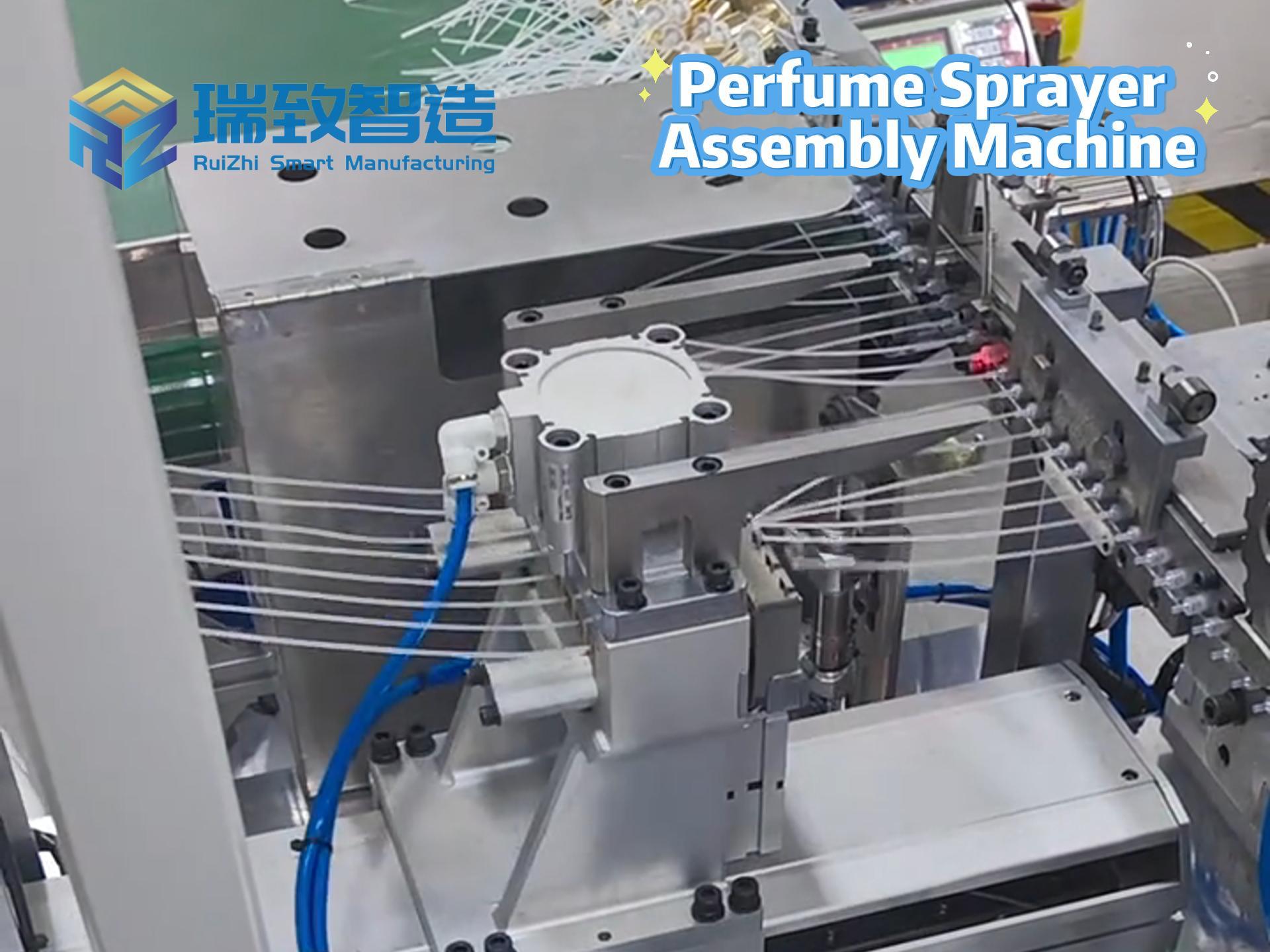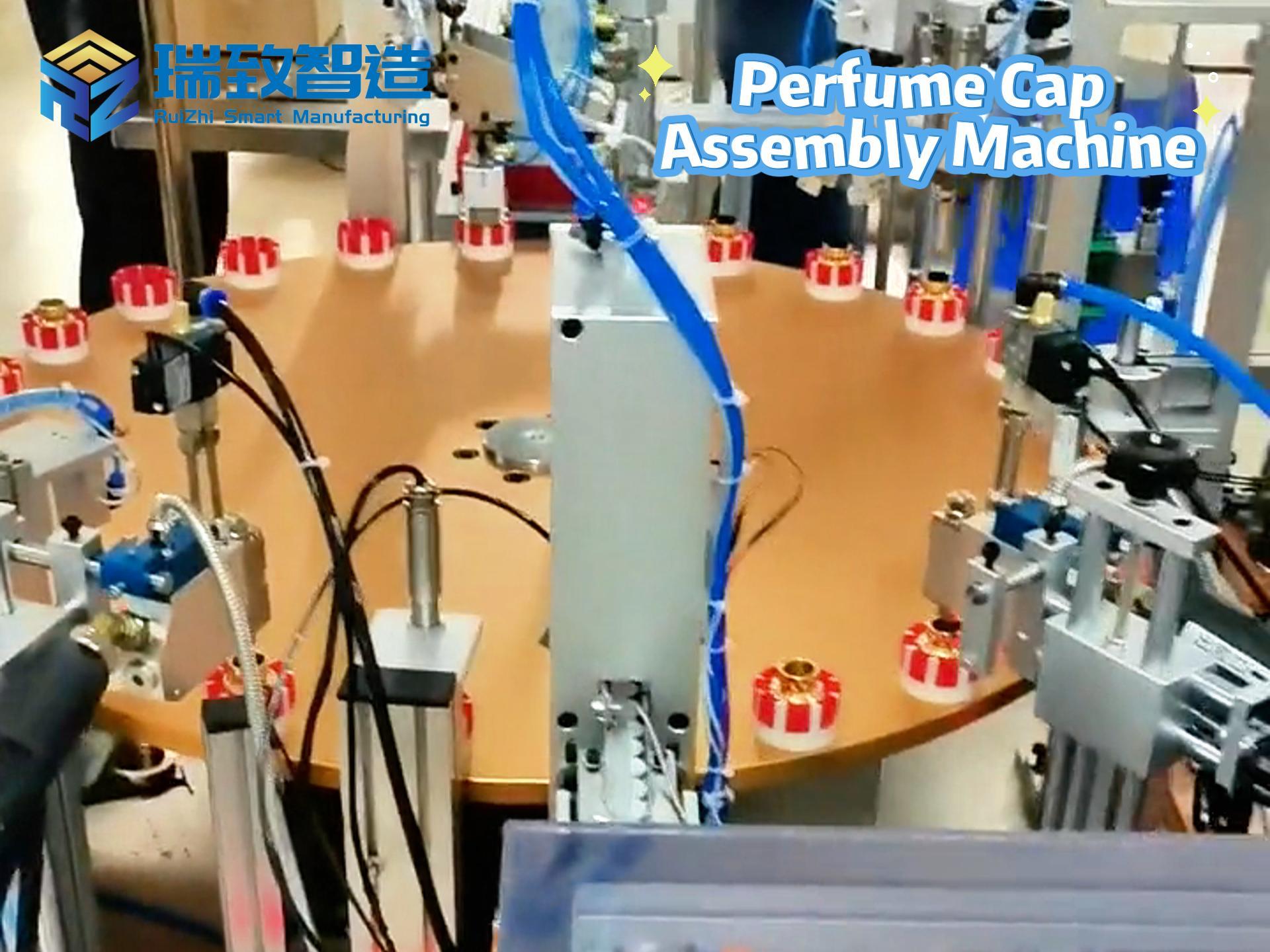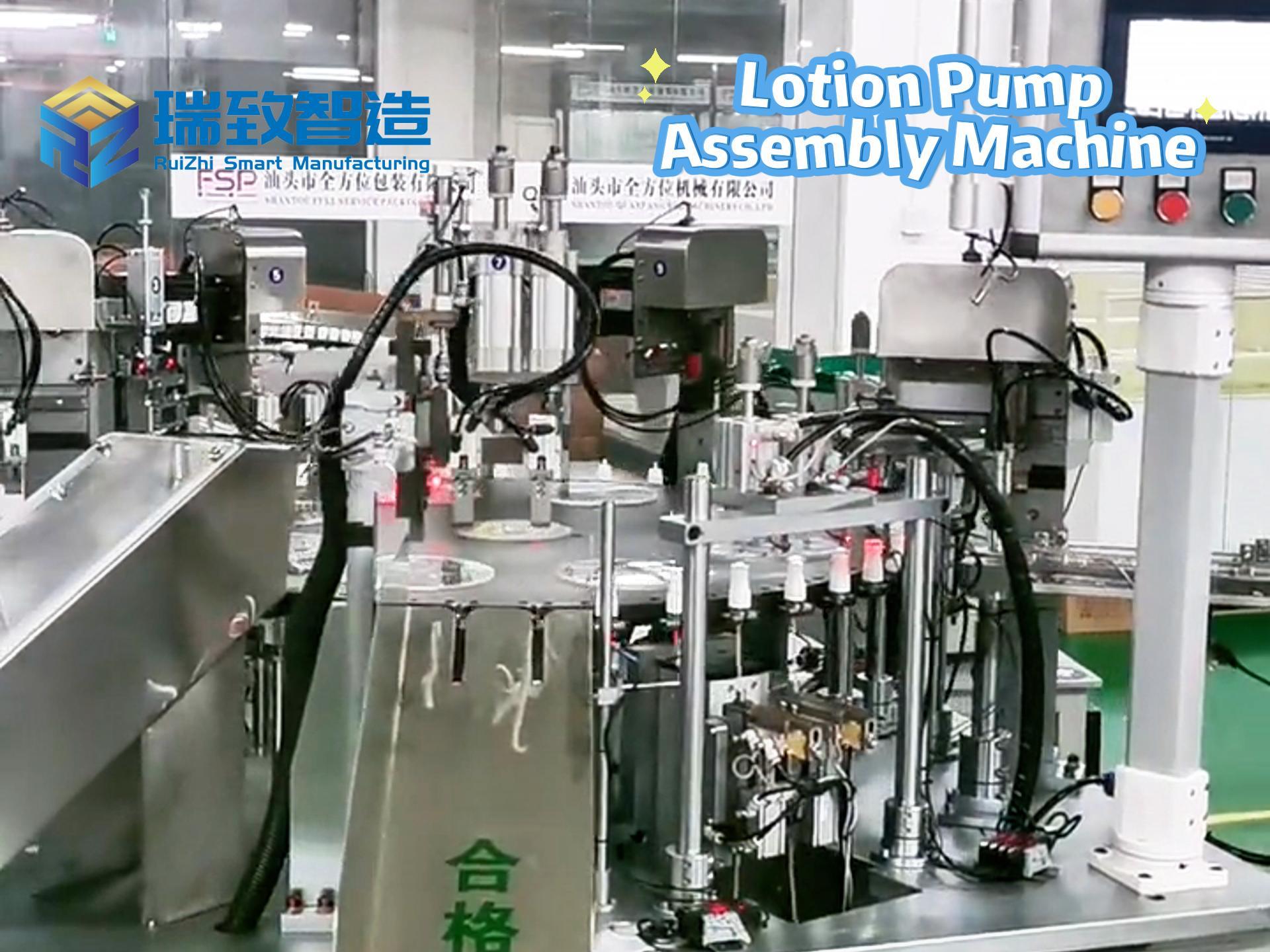
In the medical device field, “compliance” and “efficiency” are like the two ends of a balance. On one hand, it is necessary to meet the stringent verification requirements of regulatory agencies such as the FDA for the entire production process. On the other hand, it is essential to cope with the production capacity pressure brought about by the surging global medical demands. Moreover, the limited space in cleanrooms makes it even more difficult to expand production capacity. However, a manufacturer specializing in wearable monitoring devices and intelligent drug delivery systems has doubled its production capacity and reduced the unit part cost by 40% with the help of Rockwell Automation’s MagneMover Lite intelligent conveyor and modular assembly solution, without expanding the area of the cleanroom or changing the verified processes. This has provided a benchmark case for the “lean production expansion” in the medical device industry.
Industry Background: Manufacturing Dilemmas Amid Surging Demands
In recent years, the global demand for medical devices has entered a period of explosive growth. On one hand, the aggravation of population aging and the rise in the demand for chronic disease management, combined with the popularization of telemedicine and precision medicine technologies, have led to the market size of devices such as wearable heart rate monitors and intelligent insulin pumps growing at an average annual rate of over 15%. On the other hand, the pursuit of “patient accessibility” by the medical system requires manufacturers to control costs to reduce the prices of end products while ensuring accuracy and compliance.
Data from Deloitte’s 2025 Life Sciences Industry Research shows that nearly 60% of medical device manufacturing executives list “optimization of the operating model” as the core task of the year, and another 30% focus on “cost reduction and efficiency improvement”. Behind this are two major dilemmas commonly faced by the industry:
Space Constraints: Medical device production needs to be carried out in Class 8 and above cleanrooms. The cost of renovating or expanding a cleanroom can be as high as tens of thousands of yuan per square meter, and the cycle can be as long as 6 – 12 months, making it difficult to quickly respond to market demands.
Compliance Pressure: From part assembly, epoxy curing to final inspection and packaging, each step needs to pass the FDA’s Process Validation. Any adjustment to the production process requires re – submission of verification materials. The traditional “adding machines and expanding factories” extensive production expansion model is likely to break the compliance balance.
Bottlenecks of Traditional Solutions: Insurmountable Limitations of Belt Conveyors
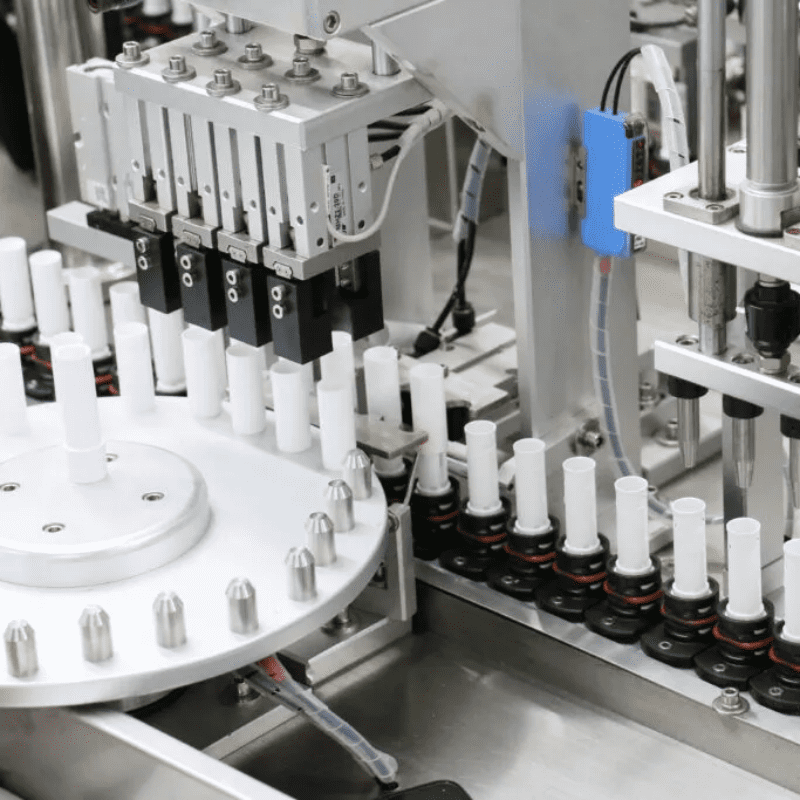
To break through the production capacity bottleneck, the above – mentioned manufacturer once tried to shift from manual assembly to automated production and jointly built a modular assembly system based on traditional belt conveyors with Automation NTH, an automation solution provider focusing on high – precision discrete manufacturing. Although this system completed the “automation transformation”, it exposed fatal shortcomings in the continuous upgrade of production capacity:
Lack of Flexibility: Belt conveyors adopt a fixed transmission path. The 30 core processes (such as fine wire bending, precision welding, and epoxy dispensing and curing) need to flow in a fixed order. If there is a short – term congestion at a certain workstation (such as an extension of the epoxy curing time), the entire production line will come to a standstill, unable to dynamically adjust the part transmission path.
Precision and Cleaning Problems: Belt conveyors are prone to vibration during operation, resulting in the bending error of fine wires exceeding the compliance standard of ±0.1mm. Moreover, epoxy glue and metal debris are likely to remain at the joints of the conveyor belt. Cleaning requires shutting down the machine for more than 4 hours, which not only affects efficiency but also increases the risk of cleanroom pollution.
Low Space Utilization: Traditional belt conveyors need to reserve a large number of buffer areas to avoid part collisions. The entire set of systems occupies 85 square meters of the cleanroom area, leaving almost no room for expansion.
When the manufacturer planned to increase production capacity by 50%, the traditional belt conveyor system could no longer meet the demand at all. Automation NTH realized that only by innovating from the “underlying transmission technology” could the contradiction between “production expansion and compliance, efficiency and space” be solved.
III. Intelligent Solution: The “Magnetic Drive Revolution” of MagneMover Lite
After comparing a variety of automated transmission technologies, Automation NTH finally chose Rockwell Automation’s MagneMover Lite intelligent conveyor as the core carrier and built a trinity solution of “hardware + software + flexible process” around it:
Magnetic Drive Carriers: Breaking the Shackles of Path and Precision
Different from the “overall drive” of traditional belt conveyors, MagneMover Lite adopts an independent magnetic drive carrier design. Each tray carrying parts is driven by a built – in magnet and can start, stop, change speed, and turn independently on the guide rail. It can even dynamically adjust the transmission path according to real – time production data (such as the process completion rate of a certain workstation):

When there is a backlog at the epoxy curing workstation, the system can automatically divert subsequent parts to the standby inspection workstation to avoid production line congestion.
For the precision requirements of different parts (for example, wire bending requires low – speed and stable transmission, and parts can be transferred at high speed after final inspection), the carrier can independently adjust the speed (0.1 – 1.5m/s) and acceleration, and control the transmission error within ±0.05mm, far exceeding the FDA’s transmission precision requirements for precision components.
Proprietary Software: The Bridge Connecting “Technology” and “Compliance”
To meet the compliance requirements of medical devices, Automation NTH has developed a proprietary software engine, which is connected to the controller of MagneMover Lite at one end and the manufacturer’s MES (Manufacturing Execution System) at the other end, realizing three core functions:
Precise Control: Real – time regulation of the acceleration, speed, and workstation allocation of the carrier to ensure that the connection time error of 30 processes does not exceed 2 seconds, avoiding compliance risks caused by manual intervention.
Data Traceability: Automatically record the transmission path, residence time, and corresponding carrier number of each part, and generate electronic records that meet the FDA 21 CFR Part 11 standard without manual data re – entry.
Flexible Switching: Supports the dual modes of “fully automated + human – machine collaboration”. When customized devices (such as small monitors for children) need to be produced, the system can quickly switch to the “robot loading + manual fine – tuning” mode without re – verifying the entire process.
Space Optimization: “Extreme Excavation” of Cleanroom Utilization
The compact guide rail design (only 80mm wide) and three – dimensional layout ability of MagneMover Lite have reduced the floor area of the entire set of assembly systems from 85 square meters of traditional belt conveyors to 52 square meters, with a 38% reduction in space occupation:
The guide rail can be laid along the walls and ceilings of the cleanroom to achieve “upper and lower layer transmission”. For example, lighter wire components are transmitted from the upper layer, and heavier metal casings are transmitted from the lower layer.
Cancel the buffer area of traditional belt conveyors, and the carriers achieve “zero – gap connection” through software scheduling, increasing the production capacity density per square meter of the cleanroom by 62%.
Innovation Results: The Leap from “Production Expansion” to “Quality Improvement and Cost Reduction”
After the MagneMover Lite intelligent conveyor system was put into use, the manufacturer’s production efficiency achieved a “qualitative leap”, and the core results far exceeded expectations:
Doubled Production Capacity and Improved Response Speed: Within the original cleanroom space, the production speed increased from 120 pieces per day to 240 pieces per day, doubling the production capacity directly. It can also quickly respond to sudden orders (such as the urgent demand for monitors during the epidemic), and the delivery cycle has been shortened from 45 days to 20 days.
Significant Cost Reduction and Improved Patient Accessibility: The unit part cost has been reduced by 40%, of which the labor cost (no need for special personnel to monitor the transmission process) has been reduced by 25%, and the consumable cost (reduced epoxy glue waste and conveyor belt replacement) has been reduced by 15%. The price of end – equipment has decreased by 18% accordingly, benefiting more low – income patient groups.
Worry – free Compliance and Convenient Maintenance: Within 18 months of system operation, there were no FDA compliance deviations. Moreover, the magnetic drive carriers have no wear and no cleaning dead corners. The maintenance time has been reduced from 8 hours per month for traditional belt conveyors to 1 hour per month, and the overall equipment efficiency (OEE) has increased from 72% to 91%.
Industry Insights: Intelligent Conveying is the “Invisible Infrastructure” of Medical Device Manufacturing
This manufacturing innovation driven by intelligent conveyors not only solves the production capacity problem for a single enterprise but also reveals the future development direction of the medical device industry. Under the constraints of “compliance first and limited space”, the intelligent transmission system of “hardware upgrade + software collaboration” will become the “invisible infrastructure” for enterprises to break through the growth bottleneck:
For small and medium – sized manufacturers, modular intelligent conveyors can lower the threshold of automation and achieve production capacity upgrades without large – scale factory renovations.
For large enterprises, the “data traceability” ability of intelligent conveying systems can connect the entire chain of “R & D – production – after – sales”, providing support for the mass production of personalized medical devices.
As the director of the medical industry at Rockwell Automation said: “In the future of medical device manufacturing, it is no longer ‘the more machines, the higher the production capacity’, but ‘every meter of the transmission path can create value’ – intelligent conveyors are re – defining the ‘efficiency standard’ of the industry.”