Catheters are indispensable minimally invasive medical devices, spanning cardiovascular intervention (e.g., coronary stenting catheters), urology (e.g., urinary catheters), respiratory care (e.g., endotracheal tubes), and neurointervention (e.g., cerebral aneurysm embolization catheters). With the global minimally invasive surgery market growing at a CAGR of over 10% (per Grand View Research), the demand for high-precision, sterile catheters is surging. However, catheter assembly is far more complex than conventional medical consumables: it involves flexible materials (silicone, TPU, FEP), multi-component integration (tips, lumens, guide wires, sensors), and strict sterility/biocompatibility requirements. Traditional manual or semi-automatic assembly not only struggles to meet these standards but also faces bottlenecks in efficiency and consistency. Against this backdrop, catheter assembly machines have emerged as a game-changer—redefining how catheters are manufactured by merging precision mechanics, intelligent control, and sterile design.
The Pain Points of Traditional Catheter Assembly: Why Automation Is Indispensable
Before the adoption of automatic assembly machines, catheter production relied heavily on manual operations, especially for core processes like lumen bonding, tip forming, and connector attachment. This model had three critical flaws that directly threatened product quality and clinical safety:
Precision Limitations for Complex Structures: Modern catheters (e.g., multi-lumen cardiovascular catheters) require sub-millimeter alignment—for example, the coaxiality of the guide wire lumen and drug-delivery lumen must be within 0.02mm to ensure smooth guide wire movement and accurate drug release. Manual assembly, affected by human hand tremors or visual fatigue, often results in misalignment, leading to clinical risks such as guide wire jamming or uneven drug distribution.
High Contamination Risk: Catheters come into direct contact with human tissues (e.g., blood vessels, urinary tracts), so sterility is non-negotiable. Manual assembly involves repeated human contact with components, even in Class 10,000 cleanrooms, increasing the risk of microbial contamination (e.g., bacteria, endotoxins). A 2022 study by the FDA found that 30% of catheter-related infections in hospitals were traceable to substandard assembly sterility.
Inefficiency and Unstable Output: A skilled worker can only assemble 20-50 complex catheters per hour (e.g., intravascular ultrasound catheters), and output fluctuates with operator skill and fatigue. For large-scale orders—such as millions of disposable urinary catheters for nursing homes—manual production often fails to meet delivery deadlines, especially during public health emergencies (e.g., COVID-19, where respiratory catheters were in acute shortage).
These pain points have made automatic assembly machines a “must-have” for catheter manufacturers. By replacing human hands with precision machinery and closed-loop control, they not only solve quality and safety issues but also lay the foundation for scaling production.
Core Technical Composition of Catheter Assembly Machines: Flexibility, Precision, and Sterility
A mature catheter assembly machine is a modular system tailored to the unique properties of catheters (flexible materials, diverse structures). Its core technology is built around three pillars: mechanical execution systems for gentle handling, intelligent control systems for process coordination, and sterile-integrated inspection systems for quality assurance.
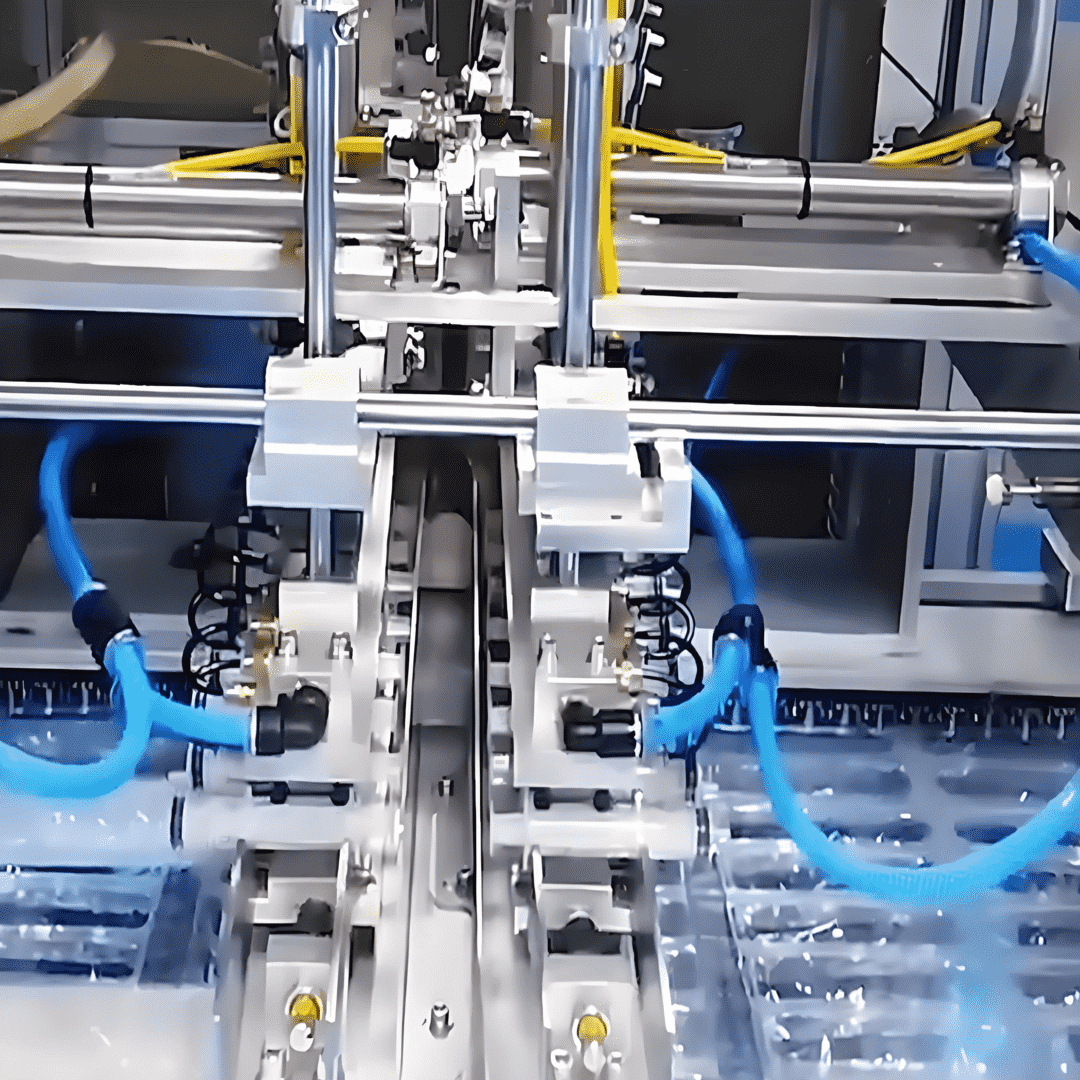
- Mechanical Execution System: Gentle Handling for Flexible Materials
Unlike rigid medical devices (e.g., syringes), catheters are made of soft, deformable materials (e.g., medical-grade silicone, thermoplastic elastomers). The mechanical system must balance precision with “gentleness” to avoid damaging components. Key modules include:
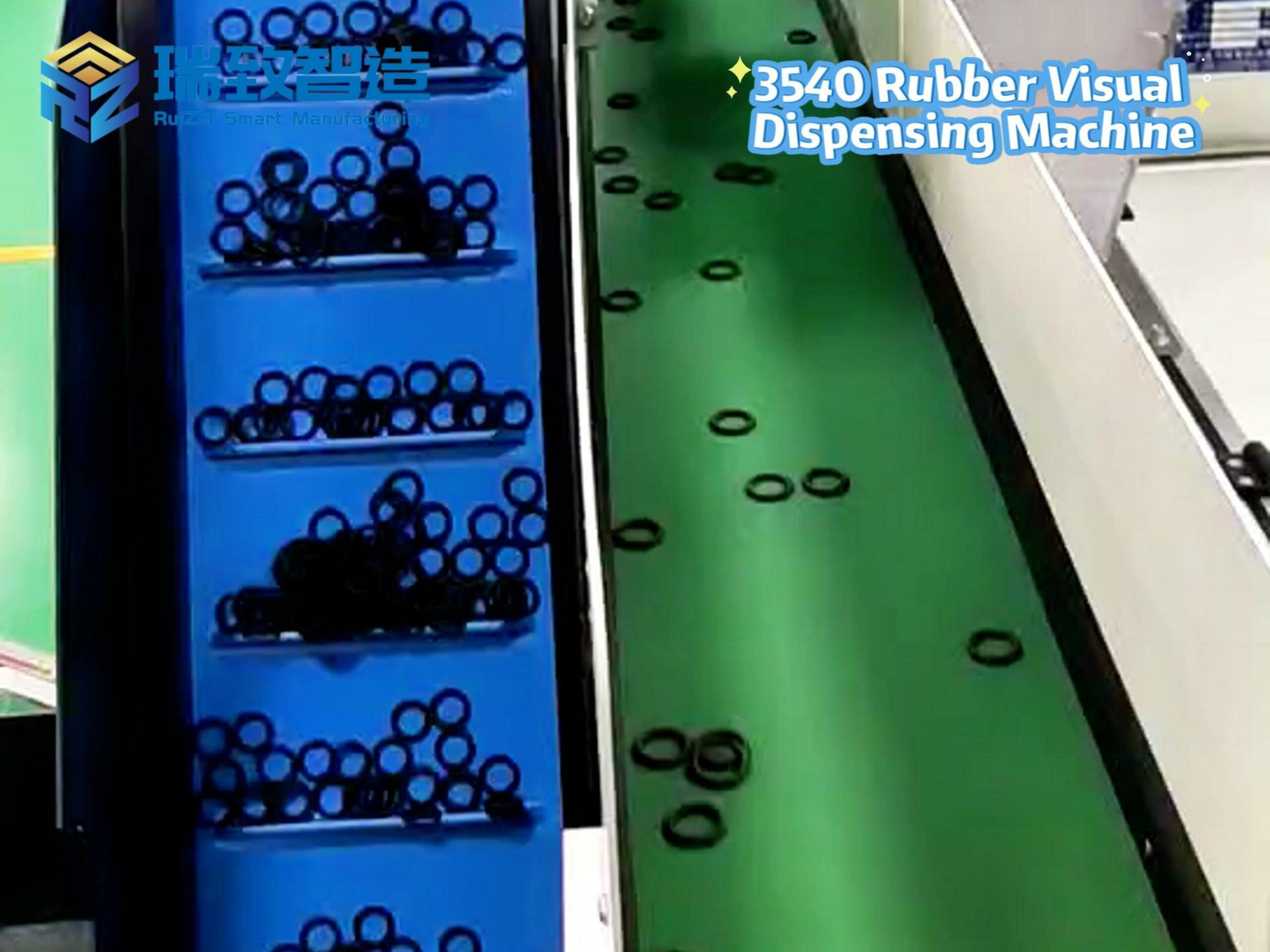
Flexible Gripping and Feeding Module:
Uses vacuum suction cups (with adjustable pressure, 0.02-0.05MPa) or soft silicone grippers to handle catheter bodies and lumens, preventing creasing or tearing. For ultra-thin catheters (e.g., 0.5mm-diameter neurointervention catheters), the module integrates micro-force sensors to monitor gripping force in real time (≤0.1N) and avoid deformation.
Adopts linear vibratory feeders with soft polyurethane rails to transport small components (e.g., Luer connectors, sealing rings), reducing friction-induced wear.
Core Assembly Modules (Tailored to Catheter Types):
Tip Forming Module: For catheters requiring atraumatic tips (e.g., cardiovascular catheters), the module uses hot-air welding (temperature-controlled at 120-180°C, depending on material) to melt and shape the catheter tip into a smooth, rounded structure—ensuring no tissue damage during insertion. The temperature and heating time are controlled by a PID (Proportional-Integral-Derivative) system with an accuracy of ±1°C.
Lumen Bonding Module: For multi-lumen catheters, the module uses laser welding (wavelength 1064nm) to bond lumen layers without adhesives (avoiding biocompatibility risks). The laser spot size is adjustable (50-200μm) to ensure precise bonding without damaging adjacent lumens.
Connector Attachment Module: Uses servo-driven pressing (force accuracy ±0.5N) to attach Luer connectors or valve components to the catheter end. The module integrates coaxiality detection to ensure the connector is aligned with the catheter lumen (deviation ≤0.01mm), preventing fluid leakage.
Material Transfer Module:
Employs magnetic levitation conveyors (instead of traditional belt conveyors) to eliminate friction between the catheter and conveyor surface. The conveyor speed is synchronized with assembly steps (50-200mm/s), ensuring continuous, stable production.
- Intelligent Control System: The “Brain” for Process Consistency
Catheter assembly involves 10+ sequential steps (feeding, forming, bonding, inspection), so the control system must coordinate modules seamlessly while adapting to different catheter specifications. Key technologies include:
PLC + HMI with Process Customization:
Uses high-performance PLCs (e.g., Siemens S7-1900) to execute logic control, such as adjusting welding temperature for silicone vs. TPU catheters. The HMI (Human-Machine Interface) supports 500+ process templates, allowing operators to switch between catheter types (e.g., urinary catheters to endotracheal tubes) in 30 minutes—far faster than semi-automatic equipment (which takes 4-6 hours).
Data Traceability and Regulatory Compliance:
Integrates with MES (Manufacturing Execution System) and RFID (Radio-Frequency Identification) technology. Each catheter is assigned a unique RFID tag, which records assembly parameters (e.g., welding time, pressure), inspector ID, and sterilization batch—meeting the FDA’s 21 CFR Part 11 (electronic records) and ISO 13485 (medical device quality management) requirements. In case of a quality recall, manufacturers can trace the affected products within 1 hour.
Real-Time Error Correction:
Equipped with torque sensors and position encoders to monitor assembly parameters in real time. If a deviation occurs (e.g., excessive pressing force on a connector), the system automatically pauses production, alerts operators, and logs the error—preventing defective products from entering the next process.
- Sterile-Integrated Inspection System: Ensuring Safety from Assembly to Delivery
Catheters require 100% quality inspection to avoid clinical risks. Automatic assembly machines integrate online inspection to eliminate manual inspection errors and ensure sterility:
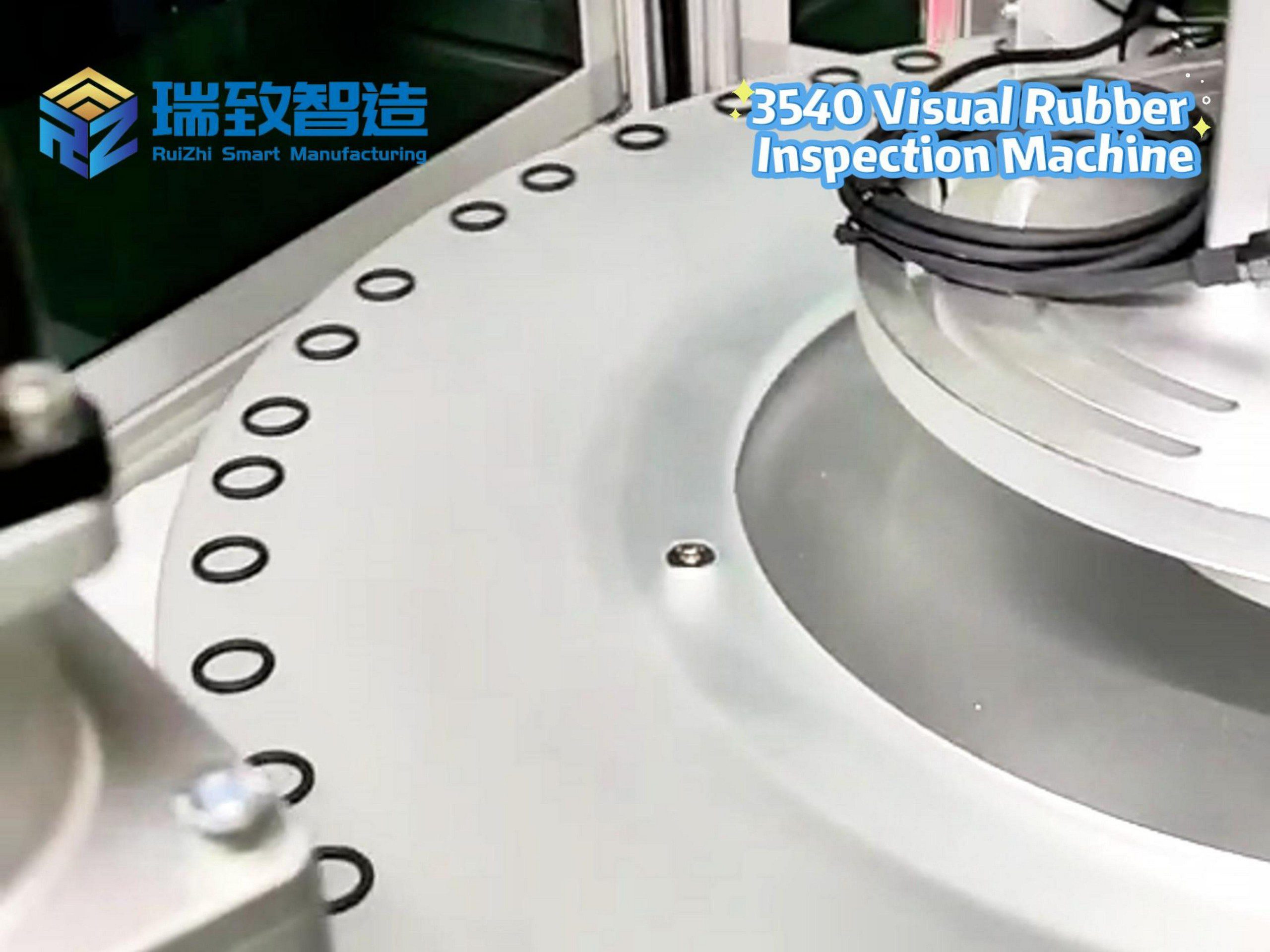
Visual Inspection for Surface and Structure:
Uses high-resolution industrial cameras (12 million pixels) and machine vision algorithms to detect surface defects (e.g., scratches on the catheter body, burrs on the tip) and structural flaws (e.g., lumen blockages, misaligned connectors). The detection accuracy reaches 0.001mm, and the rejection rate for defective products is ≥99.95%. For multi-lumen catheters, the system uses X-ray imaging to verify lumen patency and alignment—critical for drug-delivery or guide wire functions.
Leakage and Pressure Testing:
For fluid-conducting catheters (e.g., intravenous catheters), the module fills the catheter with sterile water (pressure 0.1-0.3MPa) and monitors for leaks using a pressure decay method. If the pressure drops by more than 0.005MPa in 10 seconds, the product is automatically rejected. For high-pressure catheters (e.g., angioplasty balloons), the system conducts burst pressure testing (up to 30atm) on 1 in 100 samples to ensure compliance with ISO 7886-1.
Sterility Assurance:
The entire assembly chamber is designed as a Class 100 clean area (ISO 5), with HEPA filters (99.97% efficiency for 0.3μm particles) and laminar airflow to prevent external contamination. The machine uses dry heat sterilization (180°C for 2 hours) for internal components (e.g., grippers, conveyors) between production batches—avoiding chemical sterilants that could affect catheter biocompatibility.
Core Advantages of Catheter Assembly Machines: Creating Value for Manufacturers and Patients
Compared with traditional assembly methods, automatic catheter assembly machines deliver four irreplaceable advantages:
- Precision Upgrade: Meeting the Most Stringent Clinical Requirements
Automatic machines control key parameters (e.g., welding temperature, pressing force) with sub-millimeter or sub-newton accuracy, ensuring consistent product quality. For example:
The coaxiality error of multi-lumen catheters is reduced from 0.1mm (manual) to ≤0.02mm (automatic), eliminating guide wire jamming risks.
The leak rate of fluid-conducting catheters drops from 2% (manual) to ≤0.05% (automatic), reducing the chance of infection or drug waste.
- Efficiency Leap: 10-20 Times Higher Output
A standard automatic assembly line for disposable urinary catheters can produce 500-800 units per hour—10 times the output of manual assembly. For complex catheters (e.g., intravascular ultrasound catheters), the line produces 30-50 units per hour, 5-8 times higher than semi-automatic equipment. A Chinese catheter manufacturer replaced 80 manual workers with 3 automatic lines, increasing monthly output from 50,000 to 300,000 catheters while reducing floor space by 35%.
- Compliance Assurance: Passing Global Regulatory Audits
The machine’s traceability system and sterile design help manufacturers meet strict regulatory requirements worldwide. For example:
A U.S.-based cardiovascular catheter maker used automatic assembly machines to obtain FDA approval in 6 months—half the time required for manual production lines (which often fail initial audits due to inconsistent quality).
A European manufacturer leveraged the machine’s MES integration to comply with the EU’s Medical Device Regulation (MDR), avoiding costly delays in market entry.
- Cost Optimization: Long-Term Savings Despite High Initial Investment
While automatic assembly machines have a high upfront cost ($800,000-$3 million per line), they reduce comprehensive costs by 40-60% over 3-5 years:
Labor Cost: A line only needs 2-3 operators (for monitoring and material replenishment), cutting labor costs by 80% compared with manual teams.
Scrap Cost: The defect rate drops from 5-8% (manual) to ≤0.1% (automatic), reducing raw material waste (medical-grade silicone costs $50-$200 per kg).
Maintenance Cost: Modular design simplifies repairs, and the average fault-free operation time (MTBF) is ≥8,000 hours—far longer than semi-automatic equipment (3,000-5,000 hours).
Application Scenarios: Tailored Solutions for Diverse Catheter Types
Catheters vary widely in structure and function, so assembly machines must be customized to specific use cases. Below are three typical scenarios:
- Disposable General-Purpose Catheters (e.g., Urinary, Intravenous Catheters)
Key Requirements: High speed, low cost, basic sterility.
Machine Configuration: Simplified assembly modules (feeding, tip forming, connector attachment) with high-speed conveyors (200mm/s). The inspection system focuses on surface defects and leakage.
Example: A urinary catheter assembly line produces 800 units per hour, with a total cost per unit reduced by 30% compared with manual production.
- Precision Interventional Catheters (e.g., Cardiovascular, Neurointervention Catheters)
Key Requirements: Ultra-high precision, multi-component integration (e.g., sensors, drug-delivery lumens).
Machine Configuration: Integrates laser welding (for lumen bonding), micro-force gripping (for ultra-thin catheters), and X-ray inspection (for lumen alignment). The control system supports 100+ process parameters to adjust for different catheter diameters (0.5-5mm).
Example: A coronary stent delivery catheter line achieves a lumen coaxiality error of ≤0.01mm, meeting the FDA’s requirements for interventional devices.
- Special-Function Catheters (e.g., Intravascular Ultrasound Catheters, Absorbable Catheters)
Key Requirements: Integration of electronic components, compatibility with new materials.
Machine Configuration: Adds soldering modules (for attaching ultrasound sensors) and low-temperature bonding modules (for absorbable polymers like PLLA). The inspection system includes electrical testing (to verify sensor functionality) and biodegradation performance testing (for absorbable catheters).
Example: An intravascular ultrasound catheter line assembles sensors, lumens, and connectors in one closed chamber, ensuring no electromagnetic interference with the sensor.
Industry Challenges and Future Trends: Toward Intelligence and Flexibility
Despite their advantages, catheter assembly machines face three key challenges:
High Customization Costs: Each catheter type (e.g., 2-lumen vs. 5-lumen) requires mechanical adjustments and software programming, costing $50,000-$200,000 per customization. Small manufacturers often struggle to afford this.
New Material Adaptation: The rise of absorbable catheters (e.g., PLLA-based cardiovascular catheters) and conductive catheters (for electrophysiology) requires machines to use new processes (e.g., low-temperature bonding, conductive adhesive application)—posing technical barriers for equipment developers.
Global Supply Chain Risks: Core components (e.g., precision servo motors from Japan, laser welders from Germany) are vulnerable to supply chain disruptions, extending delivery cycles from 3 months to 6-9 months.
Looking ahead, catheter assembly machines will evolve in three directions to address these challenges:
- AI-Driven Intelligent Production
AI Visual Inspection: Deep learning algorithms will improve the detection rate of subtle defects (e.g., micro-cracks in absorbable catheters) from 99.95% to 99.99%, and enable self-learning of new defect types without manual programming.
Predictive Maintenance: Sensors will monitor the wear of key parts (e.g., laser diodes, grippers) and use AI models to predict maintenance needs—reducing unplanned downtime by 60%.
Autonomous Process Optimization: The machine will automatically adjust parameters (e.g., welding temperature) based on real-time material data (e.g., silicone hardness) from inline sensors, eliminating manual intervention.
- Modular and Flexible Design
Standardized Modules: Feeding, assembly, and inspection modules will be fully standardized, allowing manufacturers to swap modules in 1 hour (e.g., switching from urinary catheter to intravenous catheter production).
Small-Batch Customization: A single line will support 20+ catheter types, meeting the demand for personalized catheters (e.g., patient-specific neurointervention catheters based on MRI scans).
- Green and Sustainable Manufacturing
Energy Saving: Uses permanent magnet servo motors and LED lighting to reduce energy consumption by 25-35% compared with current machines.
Material Recycling: Optimizes the assembly process to reduce scrap rate from 0.1% to 0.05%, and integrates a material recovery system to reuse excess silicone or TPU.
Eco-Friendly Sterilization: Replaces dry heat sterilization with ozone sterilization (lower energy consumption) and uses biodegradable lubricants to avoid environmental pollution.
Conclusion: Assembly Machines Empower the Future of Minimally Invasive Medicine
Catheters are the “lifelines” of minimally invasive surgery, and their quality directly impacts patient safety. Catheter assembly machines not only solve the pain points of traditional production but also enable manufacturers to keep pace with the rapid growth of minimally invasive medicine—from high-precision interventional catheters to eco-friendly absorbable catheters.
As AI, precision machinery, and sustainable manufacturing technologies advance, catheter assembly machines will become more intelligent, flexible, and green. They will not only be production tools but also strategic assets for manufacturers to compete in the global market—helping deliver safer, more effective catheters to patients worldwide and driving the development of minimally invasive healthcare to new heights.