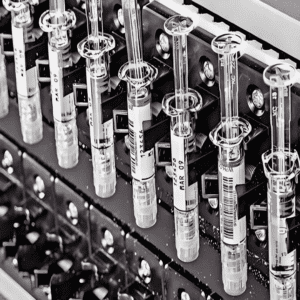
In the global medical and health system, syringes are indispensable disposable medical consumables, with demand spanning clinical injection therapy, vaccine delivery, and pharmaceutical packaging (e.g., prefilled syringes). As the world’s population ages, the expansion of immunization programs, and the rise of personalized medicine, the global syringe market size is expected to exceed $20 billion by 2028, according to Grand View Research. Behind this huge demand lies a critical transformation: the shift from traditional manual/semi-automatic assembly to full-automatic assembly equipment—a change that not only solves the pain points of low efficiency and high contamination risks in manual production but also becomes the key to meeting strict medical device regulations (such as FDA, CE, and NMPA) and ensuring product consistency.
The Pain Points of Traditional Production: Why Syringe Manufacturing Needs Automation
Before the popularization of automatic assembly equipment, syringe production relied heavily on manual operations, especially in processes such as needle tube insertion, plunger assembly, and sealing ring installation. This model had three insurmountable drawbacks:
Low efficiency and unstable output: A skilled worker can only assemble 200-300 syringes per hour, and fatigue or operational differences easily lead to fluctuations in output. For large-scale orders (e.g., millions of syringes for vaccine campaigns), manual production often fails to meet delivery deadlines.
High quality risk: Syringes have strict precision requirements—for example, the coaxiality of the needle tube and barrel must be within 0.05mm to avoid needle deviation during injection; the sealing performance of the plunger must prevent liquid leakage. Manual assembly is prone to errors such as misalignment of parts or incomplete fitting, which may cause clinical safety hazards (e.g., drug residue or infection).
Poor compliance and high contamination risk: Medical devices require compliance with GMP (Good Manufacturing Practice) standards, and manual contact with syringes increases the risk of microbial contamination. Even in clean workshops, human factors (such as skin flakes or clothing fibers) remain a major source of pollution, making it difficult to pass strict quality audits.
Against this background, syringe automatic assembly equipment has become a “must-have” for manufacturers. It integrates precision machinery, visual recognition, and intelligent control to achieve a closed-loop production process from part feeding to final inspection—fundamentally solving the bottlenecks of traditional production.
Core Technical Composition of Syringe Automatic Assembly Equipment: Precision as the Foundation, Intelligence as the Core
A mature syringe automatic assembly line is not a single machine but a modular system composed of multiple functional units, each targeting a key link in syringe production. Its core technical components can be divided into three parts:
- Mechanical Execution System: The “Hands” of Precision Assembly
The mechanical system is responsible for the accurate transfer and fitting of syringe parts (barrel, needle tube, plunger, sealing ring, and protective cap). Key modules include:
Automatic feeding module: Adopts vibration plates, linear feeders, or robotic arms to realize orderly feeding of parts. For example, the needle tube (a thin and easy-to-bend part) uses a vacuum suction + guide rail feeding method to avoid deformation; the plunger (made of rubber) uses a soft gripper to prevent damage to the sealing ring.
Core assembly module: Equipped with servo motors and precision ball screws (with positioning accuracy up to ±0.01mm) to complete high-precision operations such as needle tube insertion, plunger pressing, and protective cap capping. Taking needle tube assembly as an example, the system uses a visual positioning camera to capture the center of the barrel’s needle seat, and then the servo motor drives the needle tube to insert it at a uniform speed—ensuring coaxiality and avoiding needle breakage.
Material handling module: Uses belt conveyors or rotating turntables (with station positioning accuracy of ±0.02mm) to connect each assembly link, realizing continuous production. The turntable-type design is especially suitable for small and medium-sized syringes (e.g., 1ml insulin syringes), with a maximum speed of 120 stations per minute.
- Intelligent Control System: The “Brain” of Production Coordination
The control system is the core of the automatic assembly line, responsible for coordinating the operation of each module, collecting data, and responding to exceptions. Its key technologies include:
PLC (Programmable Logic Controller) + HMI (Human-Machine Interface): Adopts high-performance PLCs (such as Siemens S7-1500) to realize logic control (e.g., part in-place detection, assembly sequence adjustment). The HMI allows operators to set parameters (e.g., assembly speed, pressure threshold) and monitor real-time production data (e.g., output, reject rate) intuitively—supporting 100+ sets of process templates for quick switching between different syringe specifications (e.g., 0.5ml, 5ml, 20ml).
Data integration and traceability: Connects to the factory’s MES (Manufacturing Execution System) to upload production data (e.g., batch number, assembly time, inspector) to the cloud. Each syringe can be assigned a unique QR code or RFID tag, enabling full-life-cycle traceability—meeting the requirements of the FDA’s 21 CFR Part 11 (electronic records and signatures) and China’s “Measures for the Supervision and Administration of Medical Device Traceability.”
- Online Quality Inspection System: The “Eyes” of Safety Guarantee
Quality inspection is a non-negotiable link in syringe production, and automatic equipment integrates online inspection to avoid defective products flowing into the next process. Common detection technologies include:
Visual inspection: Uses high-resolution industrial cameras (up to 5 million pixels) and machine vision algorithms to detect surface defects (e.g., scratches on the barrel, burrs on the needle tube), part missing (e.g., missing protective cap), and assembly accuracy (e.g., needle tube protrusion length). The detection accuracy can reach 0.005mm, and the rejection rate for defective products is ≥99.9%.
Sealing and leakage testing: For prefilled syringes or syringes requiring high sealing performance, the system uses a pressure decay method—filling the barrel with compressed air (pressure 0.2-0.5MPa) and detecting pressure changes within 3-5 seconds. If the pressure drop exceeds 0.01MPa, the product is judged as leaking and automatically rejected.
Needle sharpness and strength testing: Samples are taken at fixed intervals (e.g., 1 in 500 products) to test the needle’s puncture force (≤0.5N for insulin syringes) and bending strength (≥10N) using a force sensor—ensuring compliance with ISO 7864 (Standard for Hypodermic Syringes).
Core Advantages of Automatic Assembly Equipment: Creating Value for Manufacturers and the Medical Industry
Compared with traditional manual and semi-automatic production, syringe automatic assembly equipment has obvious competitive advantages, which can be summarized in four aspects:
- Efficiency Leap: 5-10 Times Higher Output
A standard automatic assembly line (8-12 modules) can achieve a production capacity of 1,500-3,000 syringes per hour—5-10 times that of manual assembly. For example, a Chinese medical device manufacturer replaced 50 manual workers with 2 automatic lines, increasing daily output from 80,000 to 300,000 syringes while reducing the floor space occupied by the production line by 40%.
- Quality Stability: Reducing Defect Rate to ≤0.1%
By eliminating human factors, the automatic equipment ensures that the pass rate of syringes is stable at ≥99.9%. Taking the needle tube coaxiality as an example, the manual assembly defect rate is about 5-8%, while the automatic line can control it within 0.05%. This not only reduces the cost of scrap (syringe raw materials such as PP and stainless steel account for 30% of the production cost) but also avoids clinical risks caused by quality problems.
- Compliance Upgrade: Meeting Global Regulatory Requirements
The equipment is designed in accordance with GMP standards, with smooth surfaces (no dead corners for cleaning), food-grade lubricants, and a closed production environment (Class 100,000 clean area). The traceability system can record every link of production, making it easy for manufacturers to pass audits from regulatory authorities such as the FDA, EMA (European Medicines Agency), and NMPA. For example, a European prefilled syringe manufacturer used automatic assembly equipment to obtain the CE certificate in 3 months—half the time required for manual production lines.
- Cost Optimization: Long-Term Reduction of Comprehensive Costs
Although the initial investment of automatic equipment (about $500,000-$2 million per line) is higher than that of manual production, the comprehensive cost advantage becomes obvious in the long run:
Labor cost: A line only needs 2-3 operators (for monitoring and material supplement), reducing labor cost by 70-80% compared with manual production.
Energy consumption: Adopts energy-saving servo motors and LED lighting, with energy consumption per 10,000 syringes reduced by 30% compared with semi-automatic equipment.
Maintenance cost: The modular design makes maintenance easier, and the average fault-free operation time (MTBF) is ≥5,000 hours—reducing maintenance frequency and costs.
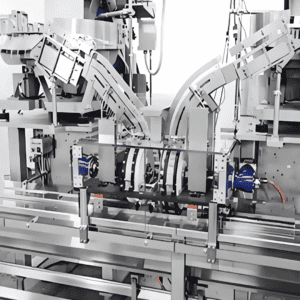
Application Scenarios: Adapting to Diversified Syringe Types
Syringe automatic assembly equipment is not a “one-size-fits-all” solution but can be customized according to the type and application of syringes. Common application scenarios include:
- Disposable Hypodermic Syringes (Most Widely Used)
Suitable for 0.5ml-50ml syringes (used in hospitals, clinics, and home care). The equipment focuses on fast assembly (up to 3,000 units/hour) and basic quality inspection (e.g., needle insertion, sealing). For example, in the context of COVID-19 vaccine distribution, many manufacturers used this type of equipment to expand production capacity, with a single line capable of supplying 5 million syringes per month.
- Insulin Syringes (High Precision Requirements)
Insulin syringes (0.3ml-1ml) have smaller specifications and stricter requirements for needle sharpness and dose accuracy. The automatic equipment is equipped with a micro-visual system (to detect needle tip burrs) and a dose calibration module (to ensure that the plunger moves smoothly and the dose error is ≤±2%). Some advanced lines also integrate insulin needle protective cap assembly to prevent needle stick injuries.
- Prefilled Syringes (Growing Demand)
Prefilled syringes (used for vaccines, biological agents, and specialty drugs) require the integration of “assembly + filling + sealing”—the automatic equipment needs to be connected to the filling machine, and the entire process is completed in a closed environment to avoid drug contamination. For example, a U.S. pharmaceutical company’s prefilled syringe line can complete assembly, filling (accuracy ±0.01ml), and rubber plug sealing in one go, with a production capacity of 1,200 units/hour.
- Special Syringes (Customized Design)
For special syringes such as safety syringes (with needle retraction function) and oral syringes (without needles), the equipment needs to add functional modules—such as a needle retraction spring assembly module for safety syringes, or a volume scale inspection module for oral syringes. This customization capability enables manufacturers to meet the differentiated needs of the market.
Challenges and Future Trends: Toward More Intelligent and Green Production
Although syringe automatic assembly equipment has become the mainstream of the industry, it still faces some challenges:
High customization threshold: Different syringe specifications (e.g., barrel diameter, needle length) require adjustments to the mechanical structure and control parameters of the equipment, which takes 2-4 weeks for general customization and even longer for complex special syringes—increasing the R&D cost of equipment manufacturers.
Rapid technological iteration of syringes: The emergence of new materials (e.g., bioabsorbable polymers) and new structures (e.g., multi-chamber syringes) requires equipment to adapt to new assembly processes. For example, bioabsorbable syringes are more fragile, so the equipment needs to use softer grippers and lower assembly pressure.
Global supply chain risks: The core components of the equipment (e.g., precision servo motors, industrial cameras) mostly rely on imports. In the context of supply chain disruptions, equipment delivery cycles may be extended, affecting the production plans of syringe manufacturers.
Looking to the future, syringe automatic assembly equipment will develop in three directions:
- Higher Intelligence: AI-Driven Autonomous Production
AI visual inspection: Integrates deep learning algorithms to improve the recognition rate of subtle defects (e.g., micro-cracks on the barrel) from 99.9% to 99.99%, and realize self-learning of new defect types.
Predictive maintenance: Uses sensors to monitor the wear of key parts (e.g., ball screws, grippers) and predicts maintenance time based on AI models—reducing unplanned downtime by 50%.
Autonomous adjustment: The equipment can automatically adjust parameters (e.g., assembly speed, pressure) according to the characteristics of parts (e.g., hardness of rubber plungers) without manual intervention.
- More Flexibility: Modular and Rapid Switching
Modular design: Each functional module (feeding, assembly, inspection) is standardized, and manufacturers can quickly replace modules according to product changes (e.g., switching from 1ml to 5ml syringes in 1 hour).
Small-batch customization: A single line can support the production of 10+ syringe types, meeting the demand for small-batch and multi-variety production (e.g., personalized drug syringes).
- Greener and More Sustainable: Reducing Environmental Impact
Energy saving: Uses permanent magnet servo motors and variable frequency drives to reduce energy consumption by 20-30% compared with current equipment.
Material saving: Optimizes the assembly process to reduce part scrap rate (from 0.1% to 0.05%) and uses recyclable packaging materials for parts.
Cleaner production: Adopts dry lubrication technology (replacing traditional lubricants) to avoid lubricant contamination and reduce the cost of cleaning the production line.
Conclusion: Automatic Assembly Equipment Empowers the High-Quality Development of the Syringe Industry
As a key link in the medical device industry chain, syringes are related to public health and clinical safety. Syringe automatic assembly equipment not only solves the pain points of traditional production but also promotes the industry to move from “quantity-driven” to “quality-driven” and “intelligence-driven.” With the continuous advancement of technologies such as AI, precision machinery, and green manufacturing, automatic assembly equipment will become more intelligent, flexible, and sustainable—helping manufacturers meet global regulatory requirements, reduce costs, and provide safer and more reliable syringes for the medical industry.
In the future, as personalized medicine and home medical care become more popular, the demand for diversified and high-precision syringes will continue to grow, and syringe automatic assembly equipment will undoubtedly become the core competitiveness of manufacturers in the global market.