For decades, the biopharmaceutical industry has been trapped in a “high-cost, long-cycle, low-success” dilemma: traditional drug discovery takes 10+ years on average, costs billions of dollars, and less than 10% of candidates advance from Phase I clinical trials to commercialization. Today, as global health demands escalate and market competition intensifies, accelerating R&D timelines, slashing development costs, and boosting clinical success rates have become not just strategic goals for biopharma companies, but existential imperatives.
Artificial intelligence (AI) has long been hailed as a “game-changer” for this industry—but not all AI is created equal. Generic AI models, trained on broad, non-specialized data, often struggle to tackle the unique complexity of life sciences: from interpreting molecular interactions to predicting patient-specific clinical outcomes. The true competitive edge, it turns out, lies in scientifically-aware AI—systems engineered to understand the nuances of biopharmaceutical research, trained on proprietary, domain-specific data (such as clinical trial endpoints, molecular structure-activity relationships, and real-world patient data) that no public or generic dataset can replicate.
This White Paper from Dassault Systèmes cuts through the AI hype to deliver actionable insights: it explores how biopharma organizations can harness the targeted power of scientific AI to reimagine every link in the R&D chain—from early-stage drug design to manufacturing scale-up—and ultimately get life-changing medicines to the patients who need them faster.
What you’ll learn
This White Paper equips biopharma leaders with clear, practical guidance on scientific AI adoption, including:
The critical divide between generic AI and scientifically-aware AI: Why domain-specificity matters in biopharma, and how proprietary data—your organization’s unique trove of clinical, molecular, and operational data—becomes an irreplaceable competitive asset.
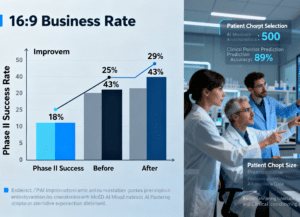
Quantified real-world successes: Concrete examples of how leading biopharma companies have leveraged scientific AI—such as a Top 10 pharmaceutical firm that boosted Phase II clinical trial success rates by 25% and improved manufacturing operational efficiency by 30%; or a biopharma supply chain leader that integrated scientific AI into its Biologische indicator assemblagemachine (equipment critical for manufacturing biological indicators, which validate sterilization in drug production). The AI system monitors real-time assembly parameters (e.g., microbial spore alignment with carrier strips, vial seal integrity) and predicts component wear, cutting assembly defects by 40% and equipment downtime by 25% while ensuring full compliance with GMP standards—directly safeguarding the reliability of drug manufacturing workflows.
Revolutionary R&D transformations: How innovations like “lab-in-a-loop” automation (where AI iterates experimental designs in real time) and multimodal AI integration (combining molecular data, imaging data, and patient EHRs) are reshaping drug discovery from a “trial-and-error” process to a data-driven science.
A phased roadmap for AI adoption: Step-by-step guidance to move from foundational readiness (e.g., unifying fragmented data infrastructure) to advanced enterprise integration (e.g., embedding AI into end-to-end R&D workflows).
Strategies to overcome key barriers: Practical solutions for addressing common pain points, including closing AI talent gaps (e.g., upskilling existing teams vs. hiring specialized roles), resolving data fragmentation (e.g., harmonizing clinical and preclinical data silos), and managing organizational change (e.g., aligning R&D and IT teams around AI goals).
Clarity on “build, buy, or partner”: A framework to evaluate whether to develop scientific AI in-house, adopt off-the-shelf solutions, or collaborate with technology partners—based on your organization’s size, R&D focus, and resource constraints.
Regulatory and compliance considerations: How to navigate the evolving global landscape for AI in regulated biopharma, including documentation requirements for AI-driven clinical decisions and ensuring data privacy (e.g., GDPR, HIPAA) in AI workflows.
Who should read this White Paper?
This White Paper is tailored for key stakeholders shaping biopharma’s digital future:
C-suite executives and board members evaluating AI investment strategies, balancing short-term ROI with long-term competitive positioning.
R&D directors and VP-level leaders tasked with modernizing drug discovery and development processes—from target identification to post-marketing surveillance.
Chief Information Officers (CIOs) and IT leaders managing data infrastructure, cloud adoption, and digital transformation initiatives that underpin AI deployment.
Founders and scientific leaders at biotechs exploring AI pilot programs (e.g., AI-driven molecular design) and building foundational capabilities for scaling.
Business development professionals assessing AI partnerships, technology acquisitions, or collaborative R&D models (e.g., biotech-tech company alliances).
Quality and regulatory affairs professionals navigating the complex, evolving requirements for AI in regulated environments—ensuring compliance without stifling innovation.
Why download this White Paper?
The biopharmaceutical industry is in the midst of its most profound technological transformation in a generation. For forward-thinking organizations, scientific AI is not a “future trend”—it is the present-day key to breaking free from the industry’s historic inefficiencies. Those that successfully integrate scientific AI into their DNA will gain an insurmountable edge: faster time-to-market for drugs, lower development risks, and the ability to tackle previously untreatable diseases. Those that delay or rely on generic AI solutions risk falling behind in an increasingly AI-driven marketplace—ultimately missing the chance to deliver life-saving therapies to patients sooner.
This White Paper bridges deep industry expertise with actionable strategy. It doesn’t just explain what scientific AI is—it shows you how to use it, with a clear roadmap to turn data into competitive advantage and transform your organization into a data-driven, scientifically aware enterprise. In the dawn of scientific AI, this is your guide to leading the next era of biopharma innovation.