Table of Contents
ToggleInnovation in Medical Automation Equipment: Dual Breakthroughs in Compliance and Efficiency

Under the strict requirements of “high precision, high compliance, and high safety” in the medical industry, medical automation equipment is no longer just an “efficiency tool” but a core infrastructure critical to patient safety and enterprise compliance. From reagent filling to surgical instrument assembly, and from sterile packaging to in vitro diagnostics (IVD) production lines, automation technologies are 破解 the industry challenge of “difficulty in balancing efficiency and compliance” through “micron-level precision control + full-process traceability + aseptic design”.
I. Technical Barriers of Medical Automation: Industry Specificity in Three Dimensions
Requirements for automation equipment in medical scenarios far exceed those in ordinary industrial fields, demanding breakthroughs in three barriers: “precision, compliance, and hygiene”:
1. Hardware Layer: Hygienic Design and Precision Control
- Materials and Structure:
- Main bodies use 316L stainless steel + food-grade engineering plastics (e.g., PEEK) with surface roughness Ra<0.8μm to prevent bacterial adhesion.
- Pipes undergo mirror polishing + electropolishing (roughness Ra<0.4μm), combined with a CIP (Clean-in-Place) system (80℃ hot water + 1% nitric acid cyclic cleaning) to ensure aseptic environments.
- Execution Precision:
- Reagent filling: Peristaltic pump + pressure sensor closed loop (precision ±0.5μL) meets micro-filling needs for insulin cartridges (0.3mL/piece).
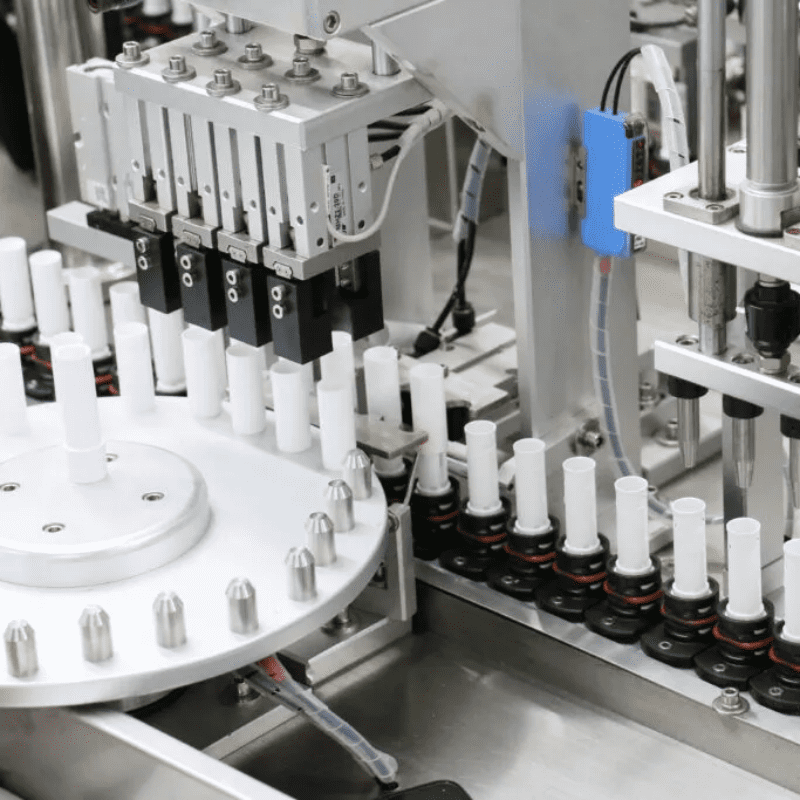
- Surgical instrument assembly: Six-axis robot + force control system (force feedback precision ±0.1N) achieves non-destructive assembly of 0.1mm-level stapler cartridge components.
2. Software Layer: Compliance Traceability and Intelligent Control
- Electronic Data Recording (EDR):
- Complies with 21 CFR Part 11 electronic signature regulations, requiring dual authorization for all parameter modifications and tamper-proof operation records (timestamp precision 1ms).
- Establishes a “product-process-equipment” full-chain traceability system, binding each reagent to a unique RFID code for traceability of 50+ parameters including filling pressure, temperature, and operator.
- Process Adaptation:
- In IVD production lines, real-time viscosity monitoring + automatic filling parameter correction (e.g., adjusting filling time by ±10ms when reagent viscosity fluctuates ±5%) ensures sampling accuracy.
- In sterile packaging lines, vision systems real-time detect sealing strength (target ≥50N), automatically rejecting and alarming for anomalies.
3. Environmental Adaptation: Dual Challenges of Asepsis and Explosion Protection
- Cleanroom Integration:
- Equipment must pass ISO 14644-1 Class 5 cleanroom certification, with laminar flow hoods (wind speed 0.45m/s±20%) at key stations to prevent particle contamination.
- Explosion-Proof Design:
- For processes involving organic solvents (e.g., ethanol, acetone) like instrument cleaning, motors and sensors must meet ATEX Zone 1 explosion-proof standards to avoid spark hazards.
II. Scenario Penetration: End-to-End Automation from IVD to Surgical Instruments
The value of medical automation delivers differentiated breakthroughs in various segments:
1. In Vitro Diagnostics (IVD) Production Lines: Micro-Upgrades with Macro-Impact
- Reagent Production:
- Nucleic acid test kit filling: Multi-channel precision dispensing systems (8/12/96 channels optional) with weighing feedback (precision ±0.1mg) achieve 0.5μL-level dispensing for PCR reagents, with CV (coefficient of variation) <5%.
- Immunochromatographic test strip assembly: Vision-guided pick-and-place robots achieve 0.1mm-level alignment of NC membrane, absorbent paper, and PVC boards, with 产能 reaching 300 strips/minute (manual only 50 strips/minute).
- Case Data: After transformation, a leading IVD enterprise reduced batch-to-batch variation from 8% to 3%, cut FDA inspection defects from 12 to 2, and increased 产能 by 60%.
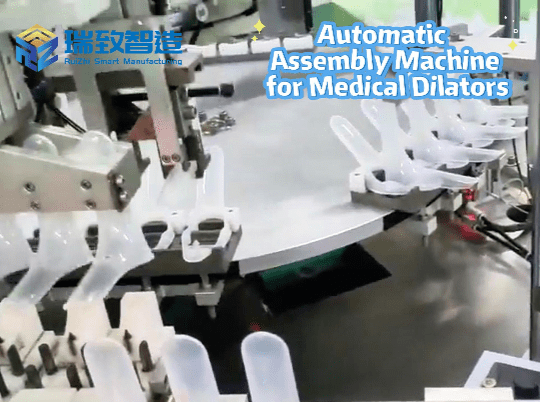
2. Surgical Instrument Assembly: “Life Protection” with Micron-Level Precision
- Stapler Production:
- Cartridge assembly: Robot + force control system maintains cartridge closing pressure within 5-8N (excessive pressure causes tissue damage, insufficient pressure leads to poor hemostasis), reducing defect rate from 1.5% to 0.2%.
- Ultrasonic knife head welding: Laser welding + infrared temperature measurement (precision ±1℃) ensures stable knife head vibration frequency at 55.5kHz±0.5kHz, preventing detachment during surgery.
- Compliance Value: A German medical device manufacturer reduced surgical instrument recall rates by 70% and shortened CE certification cycles by 40% through automation.
3. Sterile Packaging: From “Qualified” to “Traceable”
- Syringe Packaging:
- Blister packaging machines integrate online leak detection systems (vacuum decay method, detection pressure -90kPa±5kPa, alarming for leakage rate >1mL/min), replacing traditional manual water testing (low efficiency, high contamination risk).
- Labeling: Vision positioning + servo correction (precision ±0.5mm) ensures clear label information (batch number, expiration date), complying with FDA UDI (Unique Device Identification) requirements.
- Data Comparison: After automation, a syringe manufacturer reduced packaging damage rate from 3% to 0.5%, cut manual sampling from 100% to 5%, and saved ¥8 million annually.
III. Case Study: “Automated Emergency Battle” for COVID-19 Test Kits
In 2022, an IVD enterprise faced a 10-fold surge in demand for COVID-19 test kits, with traditional lines 陷入 “insufficient capacity, compliance risks”:
Pre-Transformation Pain Points
- Manual filling: Efficiency 2,000 kits/day, precision ±5μL, batch variation 10%, failing to meet urgent needs.
- Traceability gaps: Handwritten filling parameters posed risks of incomplete data during FDA inspections.
- Contamination risks: Open workstations with long reagent exposure times led to 0.8% microbial contamination rate.
Automation Solution
- Hardware Upgrades:
- 12-channel filling system (precision ±0.5μL) + weighing feedback (automatic calibration every 100 fillings).
- Fully enclosed aseptic chamber (Class 5 cleanliness) + CIP system (automatic cleaning between batches).
- Software Deployment:
- Traceability system compliant with 21 CFR Part 11, automatically recording 30+ parameters like filling pressure, temperature, and time, generating electronic reports.
- Vision inspection: Scanning reagent bottle batch numbers to bind with filling parameters for 1:1 “bottle-parameter” correspondence.
- Capacity Design:
- Dual-station parallel production with single-shift capacity reaching 20,000 kits/day (10x increase), supporting 7×24 continuous operation.
Post-Transformation Achievements
- Efficiency: From 2,000 to 20,000 kits/day, meeting emergency order demands.
- Quality: Batch variation from 10% to 3%, microbial contamination rate <0.1%.
- Compliance: Passed FDA Emergency Use Authorization (EUA) review with 100% data traceability completeness.
- Cost: Labor reduced from 20 to 5 workers per shift, labor costs down 75%.
IV. Three Key Steps for Implementing Medical Automation
From solution design to compliant operation, three challenges must be overcome: “precision, traceability, verification”:
1. Requirement Diagnosis: Compliance First, Efficiency Second
- Regulatory Benchmarking:
- Clarify target market regulations (e.g., China NMPA, EU MDR, US FDA), listing key clauses equipment must meet (e.g., FDA requirements for electronic records).
- Identify high-risk processes (e.g., aseptic filling, implant assembly) as automation priorities.
- Precision Calculation:
- Quantify process requirements (e.g., filling precision ±0.5μL) to reverse-select equipment hardware (e.g., peristaltic pump with minimum step 0.1μL).
2. Solution Design: Simulation Verification and Compliance Preview
- Cleanroom Simulation:
- Use CFD (Computational Fluid Dynamics) software to simulate cleanroom airflow (avoiding eddies causing particle accumulation) to ensure ISO Class 5 standards.
- Process Validation:
- Preview 1,000 filling processes in a digital twin system, analyzing parameter fluctuations’ impact on precision (e.g., filling volume variation <0.3μL with temperature change ±2℃).
- Compliance Pre-Verification:
- Prepare DQ (Design Qualification), IQ (Installation Qualification), OQ (Operational Qualification), PQ (Performance Qualification) documents in advance to ensure GMP compliance upon equipment delivery.
3. Debugging and Validation: From “Equipment Usability” to “Compliant Release”
- Precision Calibration:
- Calibrate filling systems with microbalances (precision 0.1mg), automatically compensating errors every 100 fillings.
- Calibrate force control systems with standard force sensors (precision ±0.01N) to ensure assembly force stays within process requirements.
- Compliance Validation:
- Three consecutive batches to verify process stability (CPK≥1.33), data traceability completeness, and electronic signature validity.
- Invite third-party agencies for GAMP 5 certification to ensure software systems meet pharmaceutical automation standards.
V. Future Trends: AI and Robots Reconstructing Medical Manufacturing
The next frontier for medical automation is the integration of “intelligence + flexibility”:
- AI process optimization: Machine learning analyzes 100,000+ batch data to automatically optimize filling parameters (e.g., predicting optimal filling pressure based on reagent viscosity), increasing yield by 2%.
- Flexible surgical robots: Multi-joint collaborative robots (e.g., single-port surgical robots in the da Vinci SP system) adapt to different surgical needs through force feedback + vision fusion.
- Decentralized manufacturing: Edge computing + blockchain enable localized production + end-to-end traceability of medical equipment in remote areas (e.g., on-site 3D printing of syringes during vaccine cold chain shortages in Africa).
The essence of medical automation equipment is “safeguarding life with technology”—it not only improves efficiency but also minimizes medical product risks through precise control and end-to-end traceability. As more enterprises break through the technical barriers of “medical compliance,” automation will shift from a “cost center” to a “quality moat,” becoming a core asset for medical enterprises in global competition.