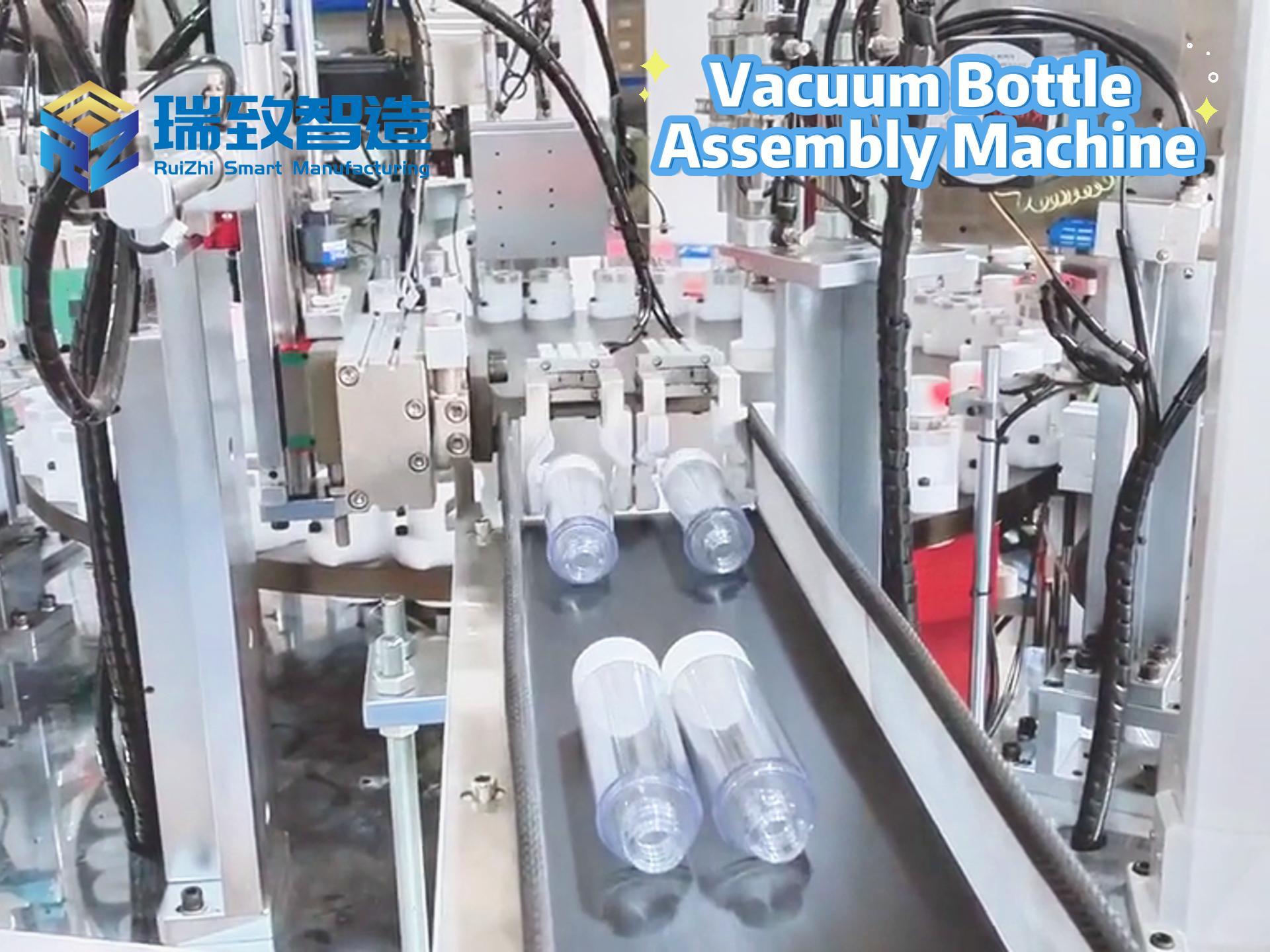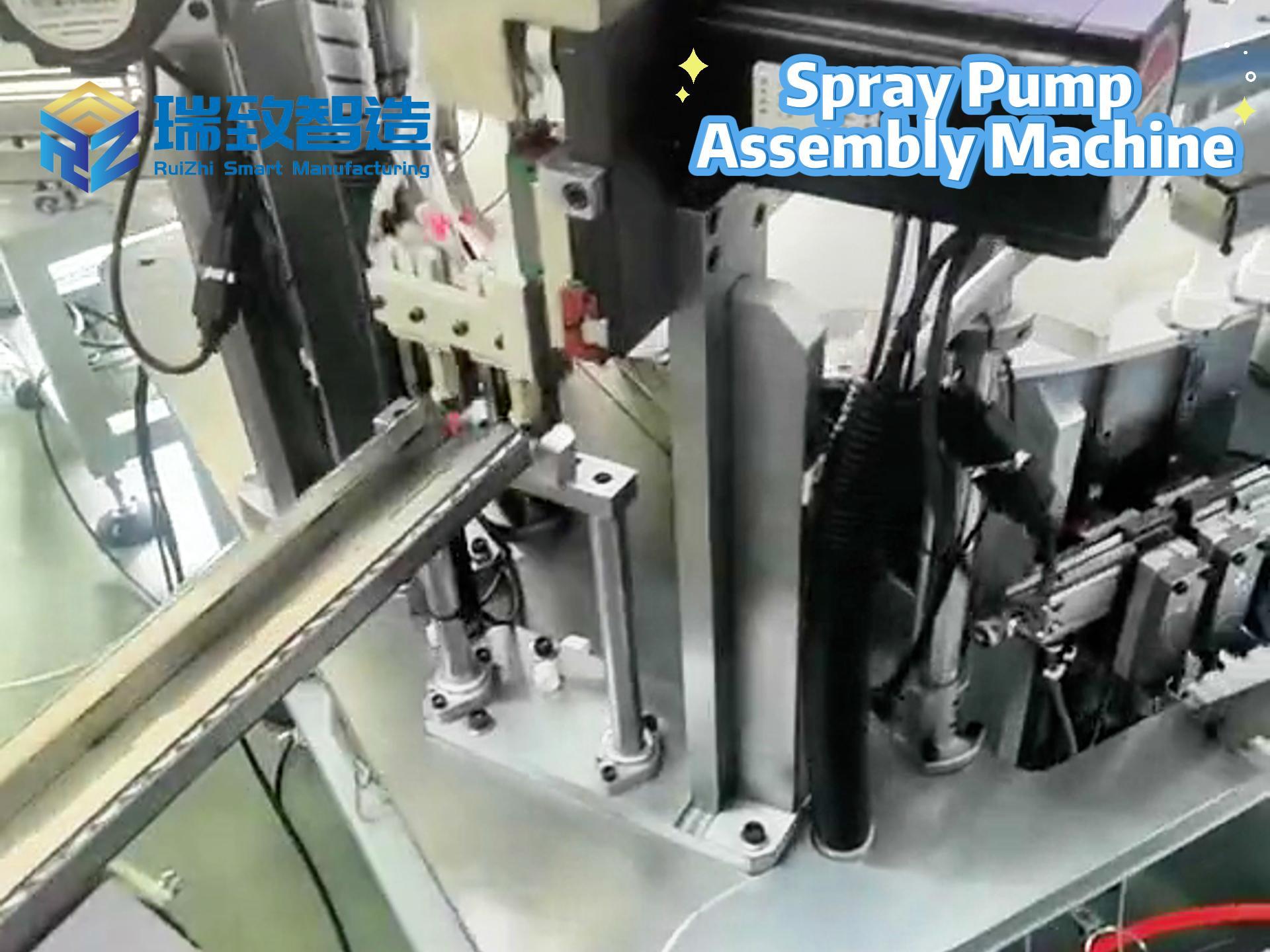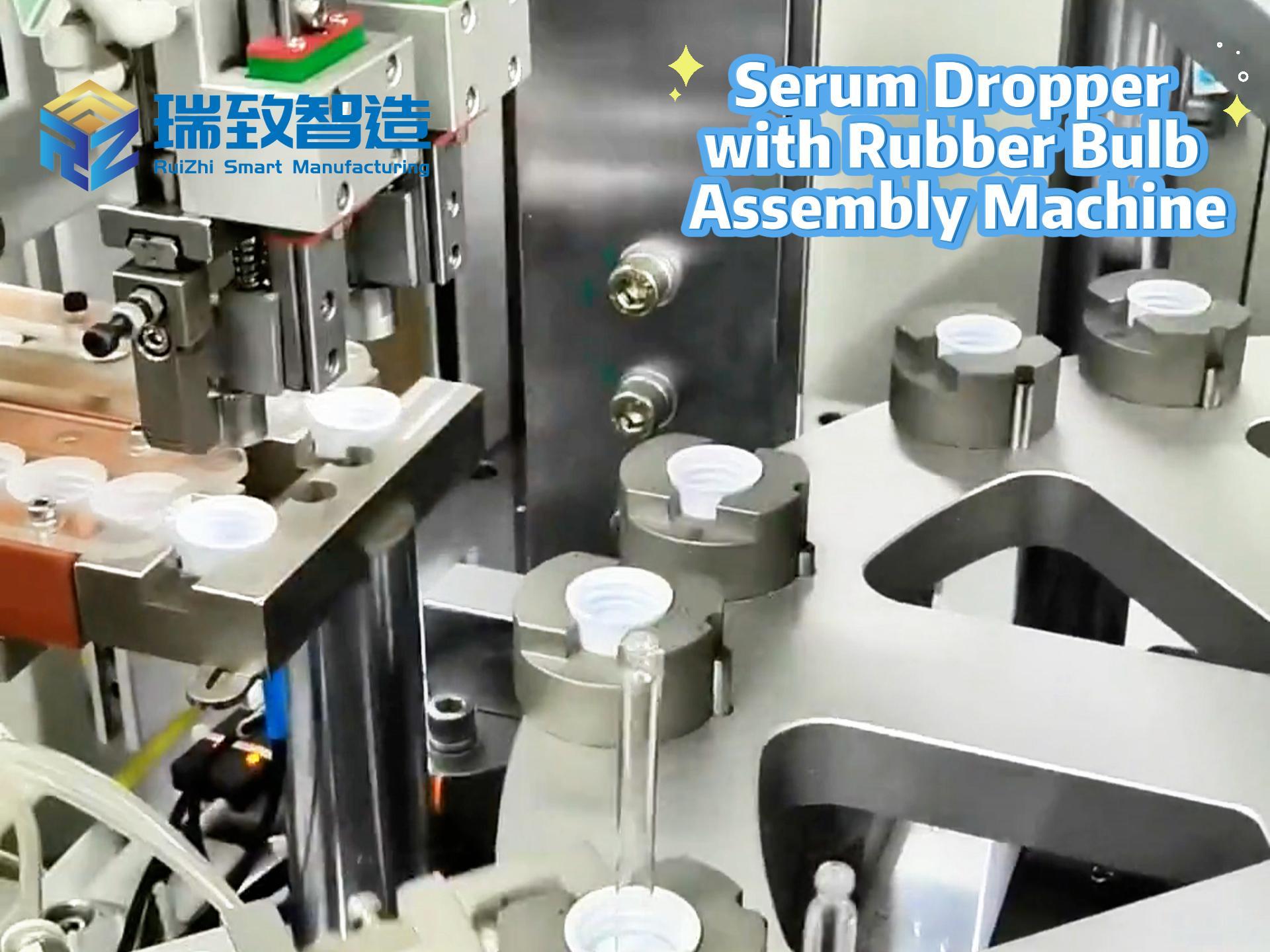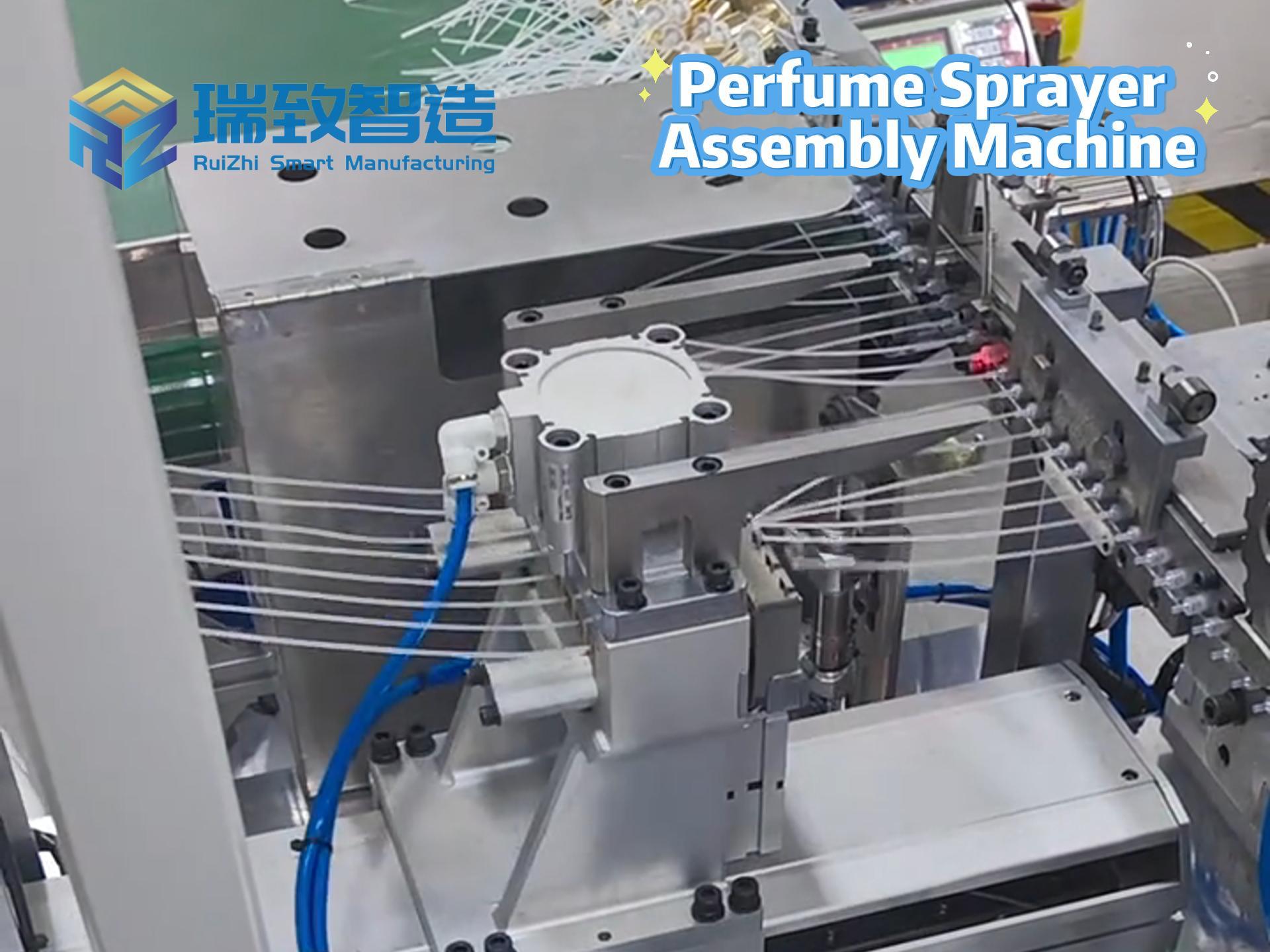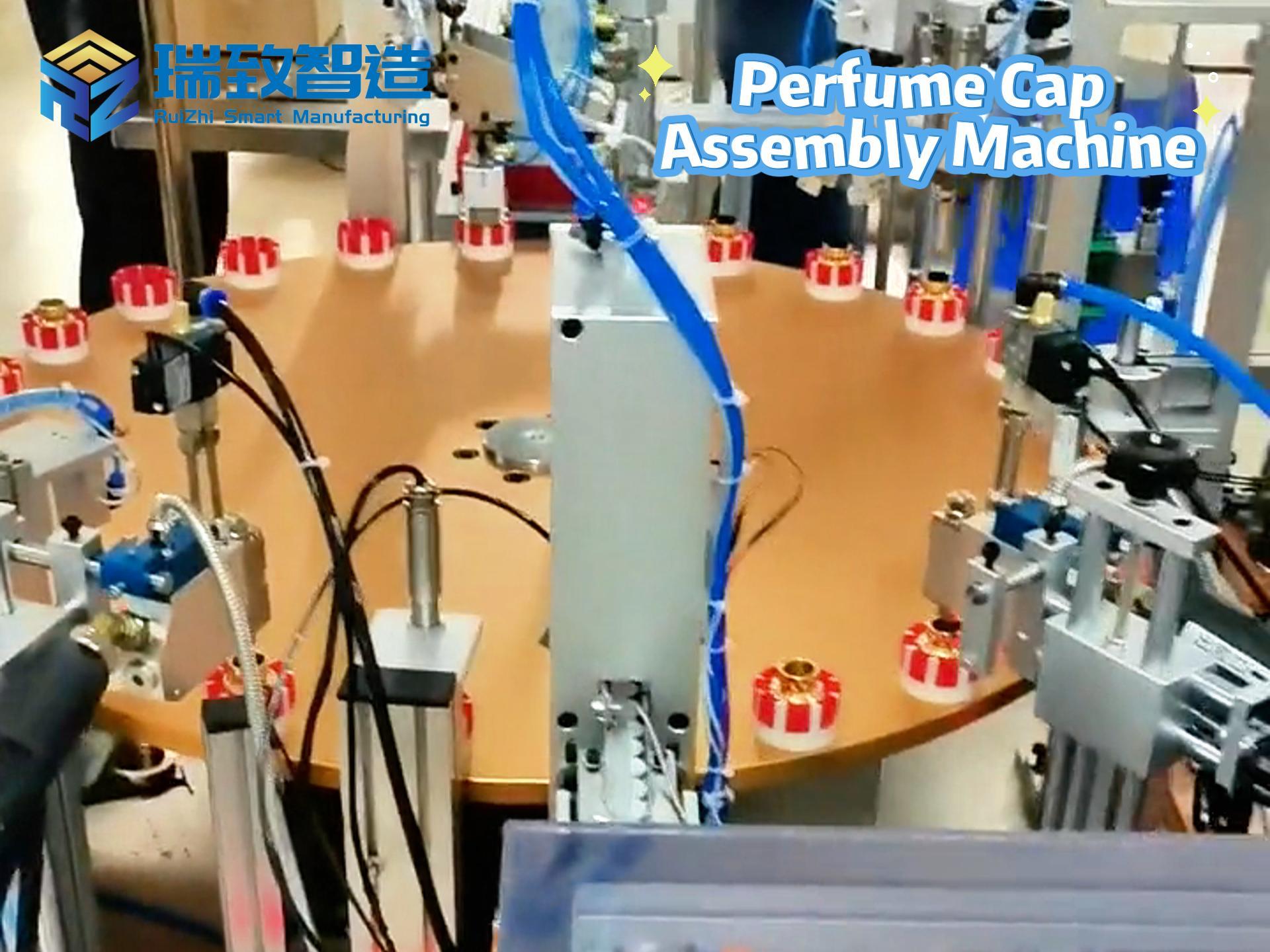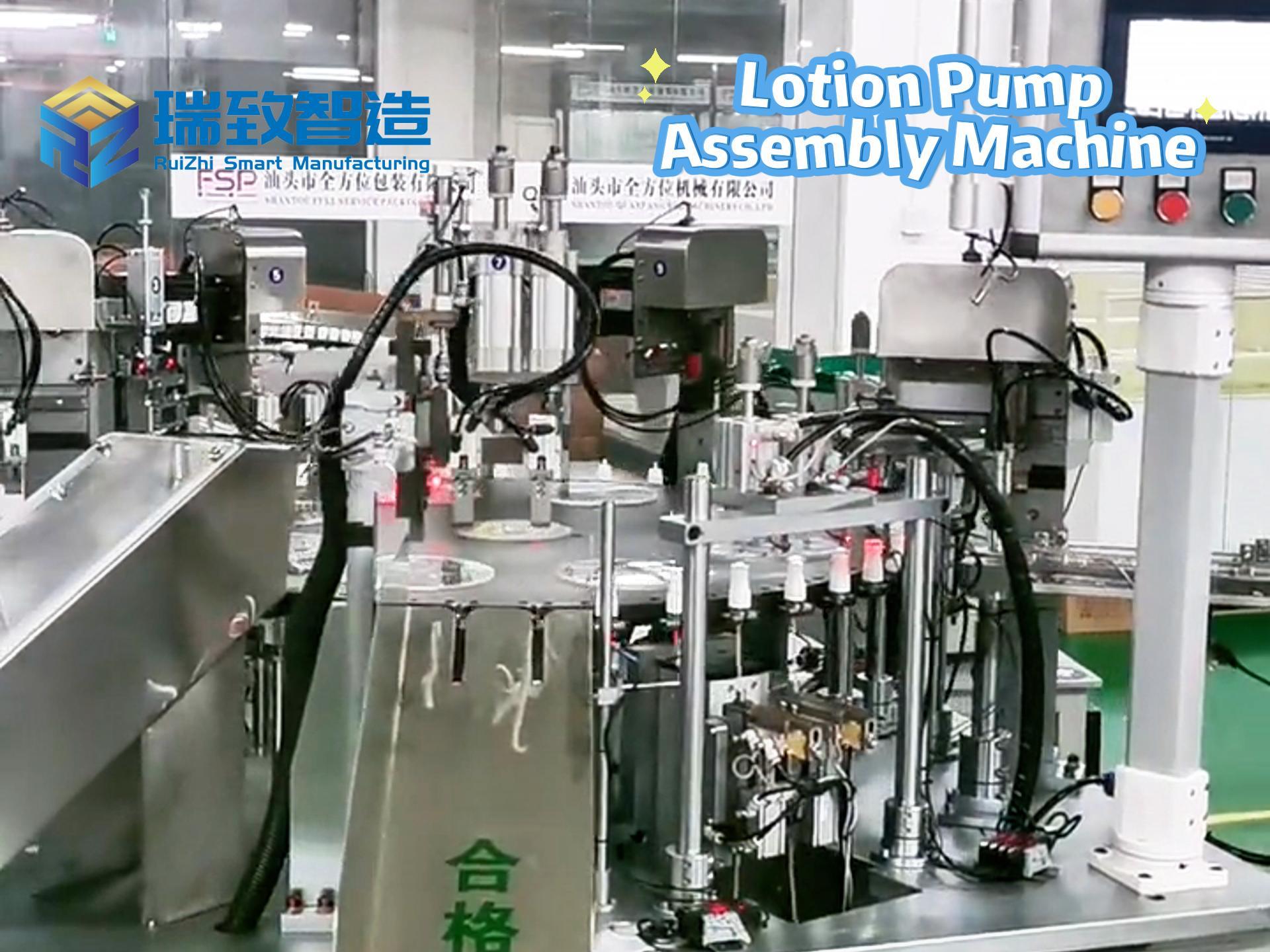
In the medical device industry, the entire process of a product from concept to market is full of technical challenges and compliance requirements. It not only needs to break through technical barriers such as micro-precision manufacturing and biocompatibility design but also meet stringent regulatory standards like FDA and ISO. Against this backdrop, Contract Development and Manufacturing Organizations (CDMOs), with their integrated “development + manufacturing” capabilities, have gradually become key partners for enterprises to accelerate product launch. Unlike traditional Contract Manufacturers (CMs) that only focus on production, high-quality CDMOs can deeply participate in the entire product development cycle and solve problems of efficiency, compliance, and scaling with a collaborative mindset. So, what specific services do CDMOs provide? And what dimensions should enterprises focus on when making a selection?
Core Services of CDMO: Beyond Manufacturing, Emphasizing Collaborative Development
The core value of CDMO lies in breaking the traditional model of “separation between development and manufacturing” and covering the entire product lifecycle from concept to mass production through integrated services. Its services can be divided into two core segments:
- Development Phase: Guided by Both Efficiency and Manufacturability
When enterprises develop independently, they often face repeated design iterations and distorted test results due to limitations in team expertise (e.g., insufficient knowledge of materials science and precision processes) or resource constraints (e.g., limited specifications of prototype materials). For example, if a complex catheter initially designed contains over a hundred components, it will not only lead to a surge in material management costs but also make it difficult to ensure mass production yield.
However, CDMOs, relying on production experience and interdisciplinary accumulation, can intervene from the prototype design stage. They provide suggestions based on material property recommendations (e.g., selection of biocompatible polymers) and design optimizations (e.g., simplifying component structures) to reduce ineffective iterations. More importantly, they integrate the concept of “Design for Manufacturability (DFM)” into the early stage of development—similar to avoiding the trap of “concept car-like complex designs” that are difficult to mass-produce—ensuring a balance between product functionality and production feasibility, thereby shortening the development cycle and reducing costs.
- Production Phase: Full-Lifecycle Manufacturing Capabilities Adapted to Complex Products
For high-precision, high-complexity advanced medical devices (such as balloon catheters and endoscopes), the production stage needs to address two major challenges: one is possible design iterations during the product lifecycle, and the other is the need for customized automation in large-scale production.
In the traditional CM model, errors are prone to occur when design plans and quality data are transferred between different organizations. However, CDMOs, through an integrated quality system, can seamlessly connect development and production. They plan automated production lines (such as semi-automatic catheter assembly equipment) from the design stage to adapt to precision manufacturing needs. In the mass production stage, relying on in-depth understanding of the product, they can quickly respond to design adjustments and ensure quality stability through Statistical Process Control (SPC). This “development-production” collaborative capability is crucial for the efficient mass production of complex medical devices.
- Three Core Considerations for Selecting a CDMO
When screening CDMO partners, enterprises need to evaluate around three dimensions: “technical adaptability, quality reliability, and supply chain resilience,” with specific focus on the following key points:
- Design for Manufacturability (DFM) Capability: The Core of Balancing Functionality and Mass Production
DFM is key to achieving “high-quality, low-cost” mass production. A high-quality CDMO should be able to intervene from the early design stage and optimize product structure based on its manufacturing experience—for example, simplifying a complex catheter with over a hundred components through modular design or customizing semi-automatic equipment to improve assembly efficiency. During evaluation, it is essential to examine whether they have full-process cases of “design to mass production” for products of similar complexity, verifying their practical capabilities in material selection, assembly logic optimization, and automation scheme design.
- Quality System: Fundamental Guarantee for Compliance and Stability
The quality of medical devices is directly related to patient safety, so the quality system of a CDMO must meet multi-level requirements:
- Basic certification: ISO 13485 certification is the minimum standard to ensure that its quality control complies with general standards in the medical device industry.
- Process control: Tools such as SPC should be used to establish risk-oriented quality checkpoints, enabling traceability and continuous improvement of the production process.
- Compliance capability: Strictly adhere to FDA document control requirements to ensure the completeness of documents throughout the process from design to post-market monitoring, avoiding compliance risks caused by material replacement or supplier changes.
- Supply Chain Management: Dual Guarantee for Cost and Delivery
Efficient supply chain management can reduce costs and minimize delays. During evaluation, attention should be paid to the CDMO’s material sourcing capabilities: whether it can stably obtain high-quality raw materials (such as medical-grade polymers and precision metal components); whether it has established an alternative supplier system to cope with sudden shortages; and whether it can optimize costs through large-scale procurement or localized supply chains, ultimately supporting the cost-effectiveness of products.
Additional Key Factor: One-Stop Service Capability
In addition to the above core dimensions, “one-stop service” capability can significantly improve cooperation efficiency. A high-quality CDMO can cover the entire process from early R&D, prototype production, clinical trial sample manufacturing to large-scale mass production, and even assist in planning the development path for next-generation products. This integrated service not only reduces cross-organizational communication costs but also provides enterprises with full-cycle experience support—for example, helping improve production strategies in the early stage to enhance financing persuasiveness, and advancing the development of next-generation products during mass production to accelerate iterative launch.
Selecting the right CDMO is essentially about medical device enterprises finding “technical collaborators and risk-sharing partners.” In the medical device industry with accelerating technological iteration and increasingly strict regulations, CDMOs with DFM capabilities, reliable quality systems, resilient supply chains, and one-stop service experience will become key enablers for enterprises to break through development bottlenecks and achieve efficient mass production, ultimately promoting more innovative medical devices to benefit patients faster and more safely.
What is the market size and development trend of medical product assembly machines?
What are the advantages of medical product assembly machines?