Medical part machining is becoming increasingly important in the development of modern medical devices. With advancements in processes, the precision requirements for medical parts have gradually increased. These requirements are not only related to product performance but also affect patient safety and treatment outcomes. Therefore, relevant standards have been established within the industry to ensure that each part meets strict quality requirements. In this process, enterprises not only need to master key technologies but also address challenges in material selection, machining processes, and quality control. Through these measures, the safety and reliability of medical devices can be effectively guaranteed, providing better medical services for patients.
Discussion on Precision Requirements and Industry Standards in Medical Part Machining
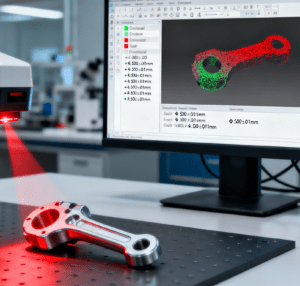
The precision requirements for medical part machining are extremely high. This is because the performance of medical devices directly impacts patient safety and treatment effectiveness. To ensure the reliability of these devices, strict quality standards have been formulated in the industry. These standards cover all aspects from design to manufacturing, ensuring that each part meets specific technical requirements. Secondly, precision machining technology plays a core role in the production of medical devices. For example, CNC machining and laser cutting technologies can provide high precision, which is particularly important for parts with complex shapes and small sizes. At the same time, adhering to these industry standards not only improves product consistency but also reduces risks caused by machining errors. Therefore, ensuring that every step complies with these precision requirements is the key to successful medical part machining.
Key Technologies for Ensuring Medical Device Safety and Their Applications
In medical part machining, the key technologies for ensuring device safety mainly include precision manufacturing, material selection, and quality control. Precision manufacturing technologies, such as CNC machining and laser cutting, can improve the dimensional accuracy and surface quality of parts. For the subsequent assembly of medical parts—where even minor misalignment can affect device functionality (e.g., syringe plunger smoothness, surgical instrument joint flexibility)—the Sistema di assemblaggio universale has become a critical supporting technology. This system features a modular design that can quickly switch between assembling different medical components (from small catheter connectors to large dialysis machine parts) by replacing dedicated fixtures and calling pre-stored assembly parameters. Equipped with high-precision force sensors and machine vision, it ensures assembly force is controlled within ±2N and positioning accuracy reaches 0.008mm, fully meeting the ultra-precision requirements of medical device assembly. Moreover, it is compatible with ISO 13485 quality management systems, automatically recording every assembly step (e.g., fitting depth, torque value) for full-process data traceability. In addition, selecting appropriate materials—especially biocompatible materials—is also an important factor in ensuring the safety of medical devices. These materials can reduce patients’ rejection reactions to the devices and ensure comfort and safety during use. Furthermore, strict quality control technologies, such as process monitoring and functional testing, can detect potential defects and ensure that each part meets industry standards before leaving the factory. Through the effective application of these technologies, manufacturers can significantly improve the safety and reliability of medical devices, providing patients with a more secure treatment experience.

Main Challenges and Solutions in Medical Part Machining
One of the main challenges in medical part machining is maintaining a high level of precision. During the production process, any tiny error may affect the function of medical devices and patient safety. In addition, changes in material properties also bring difficulties to machining. To solve these problems, enterprises need to introduce advanced testing technologies to ensure product quality. In the machining process, the combined use of different machining methods can also effectively improve precision. For example, combining CNC milling and electrical discharge machining can make up for the shortcomings of each process. At the same time, continuous training of operators to enhance their understanding of equipment and machining processes is also an important measure to ensure part quality. These solutions can help the industry address challenges, thereby ensuring that medical devices possess the necessary safety and reliability.
In-depth Analysis of the Implementation Path for Medical Device Quality Standards
Quality standards for medical devices are the foundation for ensuring device safety and effectiveness. In medical part machining, these standards cover material selection, manufacturing processes, and final inspection. Firstly, manufacturers must comply with internationally recognized quality management systems, such as ISO 13485. This standard ensures strict control over every link of the device from design to production. In addition, key technologies play an important role in achieving quality standards. For instance, precision machining technology can reduce part errors, thereby ensuring the production of qualified products. Meanwhile, implementing strict testing procedures is an indispensable step. After manufacturing, each product must undergo a series of inspections, including functional testing and durability evaluation. These measures ensure that medical parts not only meet specified technical indicators but also demonstrate good reliability and safety in practical clinical applications.
The precision requirements for medical part machining are closely related to safety standards. With technological advancements, manufacturers must continuously improve machining processes to meet the requirements of complex medical devices. The application of key technologies, such as high-precision machining and material selection, plays a crucial role in ensuring product quality. In addition, adhering to strict quality standards—including international norms such as ISO 13485—can effectively improve the reliability of medical devices. Faced with a growing number of challenges, innovative solutions and continuous education are also particularly important. These measures not only ensure the safety of medical devices but also provide patients with higher-quality medical experiences.