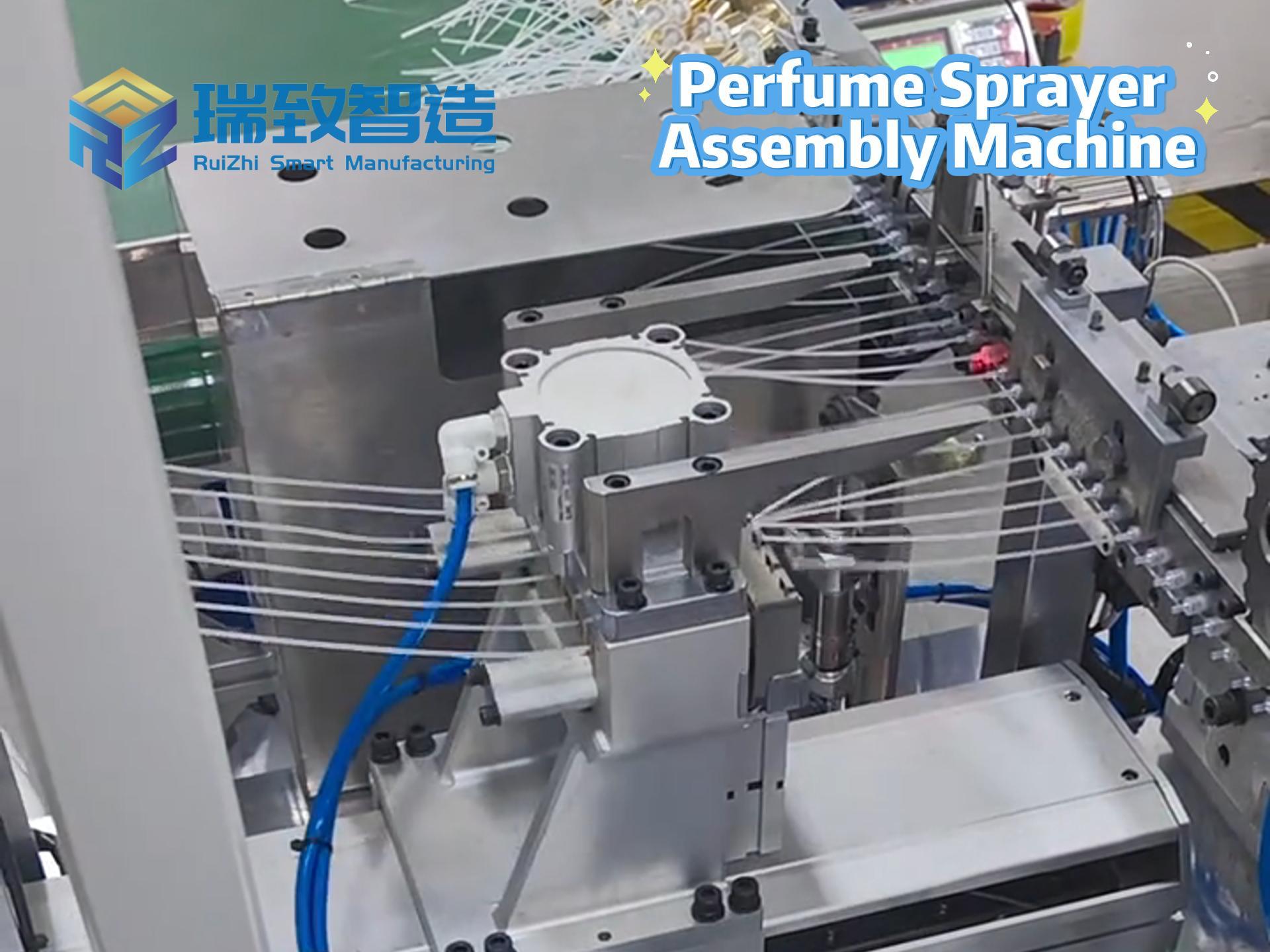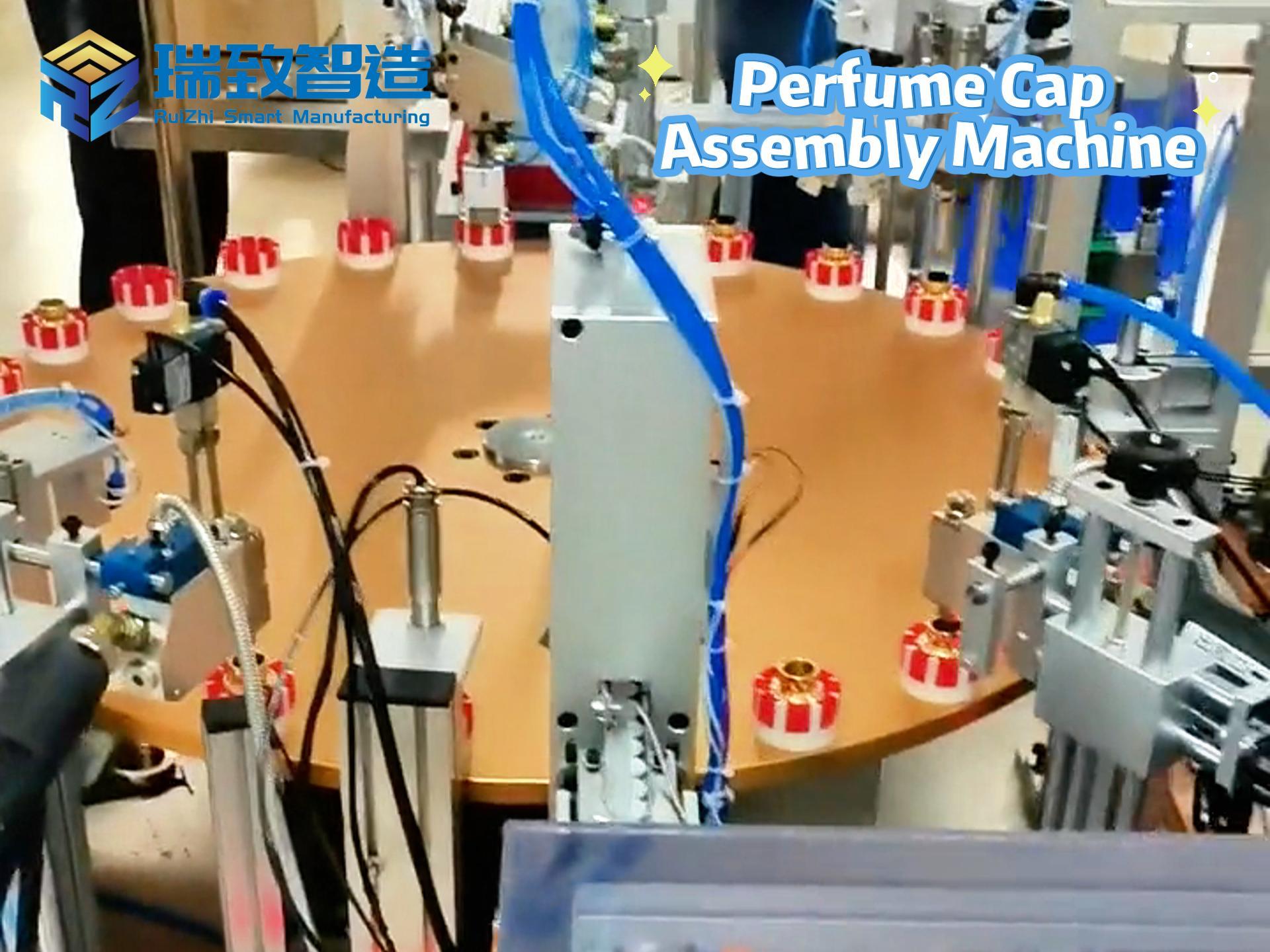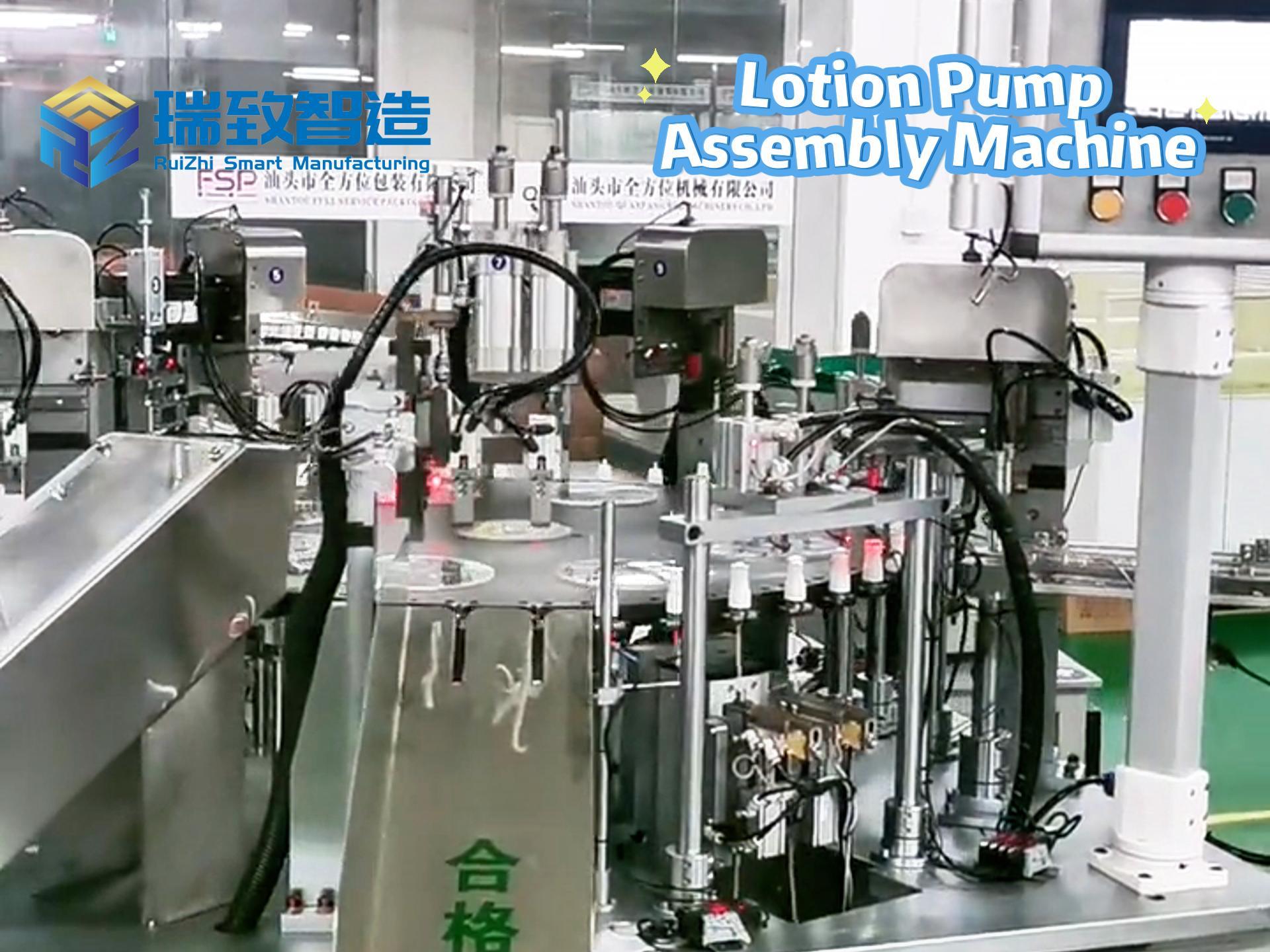
In the global medical device industry, syringes are not just “simple tools” for drug delivery—they are life-critical products that directly relate to patient safety, from vaccine injections to emergency drug administration. For decades, manual assembly dominated syringe production: workers had to manually insert plungers into barrels, attach needles, and inspect for leaks, a process that not only struggled with low efficiency (barely 300-500 units per hour per worker) but also faced high quality risks—even a tiny hair or slight misalignment could cause drug contamination or injection failures. Today, Syringe Automatic Assembly Equipment has completely rewritten this scenario, becoming the core infrastructure that supports the mass production of safe, standardized syringes worldwide.
The Evolution of Syringe Automatic Assembly Equipment: From “Semi-Automation” to “Full-Process Intelligence”
The development of syringe assembly equipment has gone through three key stages, each addressing core pain points in the manufacturing process and pushing the industry toward higher standards.
Early Semi-Automatic Equipment (1990s-2000s): Replacing Single Manual Tasks
The first generation of semi-automatic assembly machines focused on “reducing repetitive labor.” For example, some machines could automatically press needles into syringe barrels, but workers still needed to manually load empty barrels and transfer semi-finished products to leak-testing stations. This improved labor intensity but did not solve the root problems of inconsistency and inefficiency—production lines still required 3-4 workers to coordinate, and the defect rate remained around 2-3% (mainly due to manual loading errors). At the time, these machines were only suitable for low-end disposable syringes and could not meet the precision requirements of medical-grade syringes (such as those for insulin or cosmetic fillers).
Fully Automatic Assembly Lines (2010s): Integrating Core Processes
By the 2010s, fully automatic assembly lines emerged, integrating barrel feeding, plunger assembly, needle attachment, leak testing, and laser marking into a single continuous workflow. A mainstream fully automatic line could produce 1,500-2,000 syringes per hour, with the defect rate dropping to less than 0.5%. Key technical breakthroughs included:
Servo-driven precision control: Ensuring the needle is aligned with the barrel’s center axis (accuracy ±0.01mm) to avoid needle offset, which could cause pain to patients or incorrect drug delivery.
Vacuum leak testing: Using negative pressure to detect micro-leaks in syringe barrels (as small as 0.001mm), a capability far beyond manual visual inspection.
Material compatibility design: Adapting to different materials such as PP (polypropylene) barrels and TPE (thermoplastic elastomer) plungers, avoiding material deformation during assembly.
However, this generation of equipment had limitations: it could only handle 1-2 fixed specifications (e.g., 1ml or 5ml syringes), and changing molds for different sizes would take 4-6 hours—too slow to meet the “small-batch, multi-variety” needs of pharmaceutical companies (such as customized syringes for clinical trials).
Intelligent Flexible Lines (2020s-Present): Driven by Data and AI
In recent years, driven by the “Medical Device Quality by Design (QbD)” concept and Industry 4.0, intelligent flexible assembly lines have become the new mainstream. These lines integrate AI vision inspection, real-time data monitoring, and modular quick-change systems, achieving a leap in both efficiency and flexibility. For example:
Quick specification switching: By replacing only the feeding module and adjusting parameters via a touchscreen, the line can switch between 5-8 common syringe sizes (from 0.3ml insulin syringes to 50ml large-volume syringes) in 15-30 minutes.
AI-powered quality inspection: High-speed cameras capture 300-500 images per second, and AI algorithms automatically analyze defects such as plunger scratches, needle burrs, and incomplete laser markings. The defect recognition rate reaches 99.98%, far exceeding the 95% rate of traditional machine vision.
IoT-based predictive maintenance: Sensors embedded in key components (e.g., needle pressing heads, feeding rails) monitor real-time data such as pressure, temperature, and vibration. When abnormal wear is detected (e.g., the pressing head’s service life reaches 80% of its threshold), the system sends a maintenance alert, reducing unplanned downtime by 40-50%.
Core Technical Requirements: Why Precision and Safety Are Non-Negotiable
Syringes are classified as “Class II or Class III medical devices” in most countries (e.g., Class II in the U.S. FDA classification, Class III for specialized syringes like pre-filled ones), meaning their assembly equipment must meet extremely strict technical standards. Three core requirements stand out:
Ultra-High Precision: Micron-Level Control for Patient Safety
The assembly of syringes requires “micro-operations” that leave no room for error. For example:
Needle attachment force: The force to press the needle into the barrel must be controlled between 8-12N—too much force will crack the barrel, too little will cause the needle to fall off during use. Advanced equipment uses torque sensors with 0.001N precision to adjust the force in real time.
Plunger sliding resistance: The plunger must slide smoothly without jamming (resistance 1.5-3N), as excessive resistance can cause nurses to push too hard, leading to sudden drug injection and patient injury. Modern machines use force sensors to test sliding resistance for every syringe, rejecting any that fall outside the standard range.
Sterility Compliance: Avoiding Cross-Contamination
Syringes come into direct contact with drugs and human tissues, so the assembly environment and equipment must meet GMP (Good Manufacturing Practice) standards. High-end syringe assembly lines integrate:
Closed-loop dust-free design: The entire assembly process is enclosed in a Class 100 (ISO 5) cleanroom, with HEPA filters removing particles larger than 0.3μm.
Material anti-adhesion treatment: The equipment’s feeding rails and clamping parts are coated with PTFE (polytetrafluoroethylene) to prevent plastic particles from peeling off and contaminating syringes.
CIP (Clean-in-Place) systems: For equipment used in pre-filled syringe assembly, automatic cleaning and disinfection functions eliminate residual drugs or microorganisms between production batches.
Traceability: Full-Lifecycle Data Recording
In the event of a product recall (e.g., due to a batch of defective syringes), manufacturers need to quickly trace the source. Advanced assembly equipment is equipped with:
Laser marking systems: Engraving unique 2D codes on each syringe barrel, recording production date, batch number, and equipment station number.
MES (Manufacturing Execution System) integration: Real-time synchronizing assembly data (e.g., pressure parameters, inspection results) to the MES system, enabling full traceability from raw materials to finished products.
Key Application Scenarios: Beyond Disposable Syringes
While disposable syringes are the most common application, syringe automatic assembly equ
ipment has expanded into more specialized fields, supporting the innovation of medical devices:
Pre-Filled Syringes: The Fastest-Growing Segment
Pre-filled syringes (e.g., insulin pens, vaccine pre-filled syringes) eliminate the need for manual drug drawing, reducing contamination risks and improving injection convenience. Their assembly requires more precise equipment:
Drug filling precision: The equipment must inject drugs into the barrel with ±0.005ml accuracy (e.g., 1.000ml insulin cannot deviate by more than 0.005ml).
Stopper sealing: After filling, the stopper must be pressed into the barrel without generating bubbles (bubbles can cause inaccurate drug doses). Advanced lines use vacuum filling technology to remove air before sealing.
According to Grand View Research, the global pre-filled syringe market will reach $28.7 billion by 2030, and the demand for high-precision assembly equipment is growing at an annual rate of 15%.
Safety Syringes: Preventing Needlestick Injuries
Safety syringes (with retractable needles or needle shields) are designed to protect medical staff from needlestick injuries. Their assembly requires equipment to handle “moving parts”:
Retractable needle mechanism assembly: The equipment must accurately install springs and locking components into the barrel, ensuring the needle retracts automatically after use.
Functionality testing: Each safety syringe is tested for needle retraction speed and locking reliability—equipment uses high-speed cameras to verify that the needle is fully retracted within 0.1 seconds.
Specialized Syringes: Meeting Niche Medical Needs
For syringes used in ophthalmology (0.1ml ultra-small volume) or veterinary medicine (100ml large volume), assembly equipment must be customized:
Ophthalmic syringe assembly: Using micro-clamps to avoid damaging the thin barrel (thickness only 0.1mm), and laser welding to attach the needle (instead of pressing, to ensure sealing).
Veterinary syringe assembly: Reinforcing the equipment’s structure to handle thick-walled PP barrels, and increasing the needle pressing force to 15-20N (to withstand animal struggles during injection).
Future Trends: What’s Next for Syringe Automatic Assembly Equipment?
As the medical device industry evolves (e.g., personalized medicine, smart syringes), syringe assembly equipment will move toward three key directions:
Integration of “Assembly-Testing-Packaging” Full Processes
Current production lines often require transferring syringes from assembly machines to packaging lines, increasing the risk of contamination and efficiency loss. Future equipment will integrate automatic blister packaging, cartoning, and case packing into one line, realizing “one machine completes all processes”—from raw materials to finished packaged products. For example, a pre-filled syringe line could complete filling, assembly, testing, and blister sealing in a single closed loop, reducing production time by 30%.
Smart Syringe Compatibility
Smart syringes (equipped with sensors to monitor injection dose or patient blood glucose) are emerging, and assembly equipment must adapt to their complex structures:
Sensor integration: The equipment will need to accurately attach micro-sensors to syringe barrels, ensuring no damage to electronic components.
Data connection testing: After assembly, the equipment will test the communication between the syringe’s sensor and a mobile app (e.g., verifying that dose data is transmitted correctly), expanding from “mechanical assembly” to “electromechanical integration testing.”
Green Production: Reducing Material Waste
With global environmental regulations tightening (e.g., the EU’s Medical Device Regulation requiring recyclable materials), future equipment will focus on:
Material saving design: Optimizing feeding systems to reduce the waste of PP barrels (current waste rate is ~1%, which will be reduced to <0.5%).
Energy efficiency: Using servo motors with energy recovery functions and LED lighting in cleanrooms, cutting energy consumption by 20-25% compared to current models.
Conclusion: The Unsung Hero of Global Healthcare
Behind every safe syringe used in hospitals, clinics, and homes, there is the support of syringe automatic assembly equipment. It has not only solved the inefficiency and quality risks of manual production but also laid the foundation for the innovation of medical devices—from pre-filled syringes that accelerate vaccine distribution to safety syringes that protect medical staff. As the world faces public health challenges (e.g., pandemics, aging populations), the demand for high-quality syringes will only grow. For medical device manufacturers, choosing advanced syringe automatic assembly equipment is no longer an “optional investment” but a “must-have” to ensure product safety, meet regulatory requirements, and gain market competitiveness. In the future, this equipment will continue to evolve, becoming more intelligent, flexible, and green, and safeguarding global healthcare with greater precision.