In the fields of medical care, pharmaceuticals, and food processing, “sterilization” is a critical barrier to preventing microbial contamination—whether it’s surgical instruments in hospitals, injectable drugs in pharmacies, or sealed packaged food on supermarket shelves, only through strict sterilization can their safety be guaranteed. However, how to confirm that sterilization has truly eliminated harmful microorganisms? This relies on a core tool: Biological Indicators (BIs). As the “final inspector” of sterilization effects, BIs contain standardized, highly resistant microbial spores (such as Geobacillus stearothermophilus for steam sterilization). If the spores are inactivated after sterilization, it proves the process is effective; if they survive, it means there’s a risk of contamination.
Yet, BIs are not ordinary test products—their structure (including spore carriers, culture medium tubes, and airtight seals) and production environment have extremely strict requirements. Any error in assembly (such as spore contamination, incomplete sealing, or damaged carriers) will directly invalidate the sterilization test results, posing hidden dangers to public health. Against this backdrop, the Biological Indicator Assembly Machine (BIAM) has emerged as a core production equipment in the BI industry. It replaces manual operations with automated, sterile, and precision-controlled processes, ensuring that every BI meets “medical-grade” standards and becomes a reliable “guardian” of sterilization safety.
The “Unavoidable Risks” of Manual Assembly: Why BIs Cannot Rely on Human Hands
Biological Indicators are classified as Class II medical devices in most countries, and their production must comply with strict standards such as ISO 11138 (Biological indicators for sterilization) and GMP (Good Manufacturing Practice). Manual assembly, however, faces insurmountable bottlenecks in five core areas, making it impossible to meet the safety and consistency requirements of BIs:
- Sterility Risks: “Invisible Contamination” That Destroys BI Validity
BIs contain live microbial spores, but the assembly process must avoid introducing external microorganisms (such as Staphylococcus aureus or mold). Manual assembly involves direct contact between workers’ gloves, tools, and components—even in a Class 100,000 cleanroom, the risk of external contamination is as high as 15-20%. Once contaminated, the BI will show false positive results (spores survive due to external contamination, not sterilization failure), leading enterprises to misjudge sterilization effectiveness and cause safety accidents (e.g., hospital infections from unsterilized instruments).
- Spore Activity Damage: “Rough Operation” Reduces Test Accuracy
The spores in BIs have strict activity requirements (e.g., a minimum of 10⁶ colony-forming units per BI). Manual assembly often involves “rough operations” such as forceful insertion of spore carriers (e.g., filter paper strips) into culture tubes or tight capping, which can crush spores or expose them to excessive pressure, reducing their activity by 30-50%. Such BIs will show false negative results (spores are inactivated by assembly, not sterilization), making enterprises unaware of ineffective sterilization.
- Precision Defects: “Visual Judgment” Fails to Meet Micron-Level Requirements
Key BI components have tiny sizes: the spore carrier (filter paper strip) is only 5mm×15mm, and the culture medium tube has an inner diameter of 8-10mm. During assembly, the carrier must be placed in the center of the tube (offset ≤±0.5mm) to ensure uniform contact with the culture medium after activation. Manual assembly relies on visual judgment, and the offset often exceeds ±2mm—this leads to uneven spore growth during culture, making it impossible to accurately determine sterilization results.
- Consistency Bottlenecks: “Human Variability” Causes Batch Differences
A medium-sized BI manufacturer needs to produce 10,000-50,000 BIs per day. Manual assembly is affected by workers’ skill levels, fatigue, and operational habits: for example, the torque used to cap culture tubes may vary from 0.3 N·m to 0.8 N·m (the standard range is 0.5±0.05 N·m). This inconsistency leads to a batch pass rate of only 85-90%—defective products (such as loose caps that leak culture medium or over-tightened caps that crack tubes) not only waste materials but also pose risks if they flow into the market.
- Efficiency Limitations: “Slow Speed” Cannot Keep Up with Market Demand
A skilled worker can assemble only 60-80 BIs per hour, and needs to take breaks every 2 hours to avoid fatigue. For manufacturers with an annual output of 10 million BIs, manual assembly requires a team of 30-50 workers, with labor costs accounting for 40-50% of total production costs. In peak seasons (e.g., during flu outbreaks, when hospitals increase BI purchases), manual production cannot meet demand, leading to supply shortages.
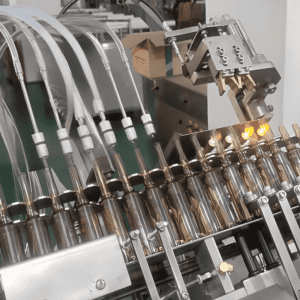
The “Five-Core Shield” of Biological Indicator Assembly Machines: How to Achieve “Sterility + Precision + Consistency”
A high-quality BIAM is not a simple combination of mechanical arms and conveyors, but an integrated system that integrates sterile design, precision control, spore protection, online detection, and data traceability. Its core competitiveness lies in five key modules, which together build a “safety barrier” for BI production:
- Sterile Feeding System: “Zero-Contamination” Supply of Tiny Components
BI components (spore carriers, culture tubes, caps, gaskets) require absolute sterility during feeding. The BIAM’s feeding system uses a “closed-loop sterile design” to avoid contact with external air:
Sterile vibratory bowls: The vibratory bowls for components are made of 316L stainless steel and can be sterilized by autoclaving (121°C, 30 minutes) before use. Customized tracks ensure components are arranged in a unified orientation (e.g., culture tube openings upward, spore carriers with spores facing inward).
Vacuum transfer channels: Components are transported from the vibratory bowl to the assembly station through a closed vacuum channel, filled with sterile filtered air (HEPA filter, 0.22μm pore size) to prevent external contamination.
Robot-assisted picking: A 6-axis collaborative robot with a sterile silicone suction nozzle picks up components—no human hands touch the parts, reducing contamination risk to less than 0.1%.
- Spore-Protective Assembly Module: “Gentle Operation” Like a “Microbiologist”
The assembly module is the “core hand” of the BIAM, and its biggest challenge is to protect spore activity while ensuring precision. It uses two key technologies:
Force-controlled insertion: A force sensor (accuracy ±0.01 N) is installed at the end of the robot arm. When inserting the spore carrier into the culture tube, the force is controlled at 0.1-0.2 N—light enough to avoid crushing spores, but firm enough to prevent the carrier from falling out.
Sequential assembly logic: The machine follows a strict process preset by ISO 11138: “Sterilize culture tube → load spore carrier → add culture medium (if pre-filled) → seal with gasket → cap and tighten”. This avoids process confusion (e.g., adding medium before sterilization) that damages spores.
- High-Precision Sealing Module: “Air-Tight” Protection Against Medium Leakage
The sealing of BI culture tubes is critical—even a tiny leak will cause culture medium to spill during transportation, making the BI unusable. The BIAM’s sealing module uses “double-layer verification” to ensure airtightness:
Torque-controlled capping: A servo motor controls the capping torque to 0.5±0.05 N·m, ensuring the cap is tight but not over-tightened (to avoid cracking the tube).
Vacuum leak testing: After capping, the tube is placed in a vacuum chamber. If the pressure rises by more than 0.5 kPa within 10 seconds, the BI is judged to be leaky and automatically rejected.
- Online Full-Inspection System: “Zero-Defect” Quality Gate for Every BI
Every BI must pass multiple inspections before leaving the assembly line—manual sampling inspection (common in traditional production) cannot cover all products, but the BIAM’s online system inspects 100% of BIs:
Visual inspection: A 20-megapixel industrial camera checks the BI’s appearance (no scratches on the tube, correct position of the spore carrier) and prints (clear batch numbers and expiration dates).
Spore activity sampling: For every 500 BIs, 1 is randomly selected to test spore activity (cultured at 55°C for 48 hours to check for growth). If activity is insufficient, the entire batch is recalled.
Culture medium volume detection: A laser sensor measures the volume of pre-filled culture medium (error ≤±0.1 mL), ensuring consistent activation conditions for all BIs.
- Sterile Environment Integration: Building a “Contamination-Free” Production Space
The entire BIAM is integrated into a closed sterile cabinet, which meets ISO 5 (Class 100) cleanliness standards—far stricter than the cleanrooms used in manual assembly:
HEPA filtration + UV sterilization: The cabinet is equipped with a two-stage HEPA filter (air change rate 60 times/hour) and UV lamps (254nm wavelength) that sterilize the internal environment for 30 minutes before production.
Negative pressure control: The cabinet maintains a negative pressure of -10 Pa to prevent contaminated air from leaking into the assembly area.
Easy cleaning design: The cabinet’s inner walls are smooth with no dead corners, and can be cleaned with 70% ethanol or hydrogen peroxide, avoiding residual microorganisms.
III. Scene Landing: From “Hospitals” to “Supermarkets”, BIAMs Empower Multi-Industry Sterilization Safety
Different application scenarios require BIs with different specifications (e.g., steam sterilization BIs for hospitals, ethylene oxide sterilization BIs for pharmaceuticals, 辐照 sterilization BIs for food). The BIAM’s flexible design allows it to adapt to diverse needs, becoming a core equipment in three key industries:
- Medical Device Industry: Ensuring Surgical Instrument Sterilization
Hospitals rely on steam sterilization (134°C, 3-4 minutes) for surgical instruments, and require BIs with high-temperature-resistant spores (Geobacillus stearothermophilus). A domestic medical device manufacturer in Shanghai adopted a BIAM in 2022: the machine assembles 300 BIs per hour, with a pass rate of 99.8% (up from 90% in manual production). The BIs are used in 50+ top hospitals, and no sterilization-related infections have been reported since application—proving the machine’s reliability.
- Pharmaceutical Industry: Guaranteeing Drug Sterility
Pharmaceutical companies use ethylene oxide (EO) to sterilize injectable drug packaging, requiring BIs with EO-resistant spores (Bacillus atrophaeus). A pharmaceutical BI manufacturer in Jiangsu customized a BIAM with a “pre-filled culture medium module”: the machine automatically injects 2mL of sterile culture medium into each tube before sealing, avoiding manual injection’s contamination risk. The machine’s output reaches 500 BIs per hour, and its data system is connected to the EU’s EudraGMDP database, meeting CE certification requirements for export to Europe.
- Food Processing Industry: Preventing Food Spoilage
Food companies use gamma-ray to sterilize sealed snacks (e.g., potato chips), requiring BIs with radiation-resistant spores (Corynebacterium radiodurans). A food BI manufacturer in Guangdong used a BIAM with a “flexible fixture design”: by replacing the vibratory bowl track and adjusting the robot’s assembly path, the machine can switch between 3 types of food-grade BIs (different tube sizes) within 20 minutes. This meets the needs of 10+ food brands, reducing the food spoilage rate caused by ineffective sterilization from 2% to 0.1%.
Future Trends: Toward “Smarter, More Compliant, and Greener”
As global regulatory requirements for sterilization safety become stricter (e.g., the U.S. FDA’s 2024 update to BI production guidelines) and the BI market expands (expected to reach $1.2 billion by 2028), Biological Indicator Assembly Machines are evolving in three key directions:
- AI-Driven Intelligent Upgrade
Future BIAMs will integrate AI vision systems that can automatically identify “micro-defects” (e.g., 0.1mm scratches on spore carriers) invisible to human eyes. AI will also predict maintenance needs—for example, alerting when the force sensor’s accuracy decreases (based on 6 months of operation data), reducing unplanned downtime by 40%.
- Global Regulatory Compliance
BIAMs will be preconfigured to meet diverse regional standards: for the U.S. market, the machine’s data system will support FDA 21 CFR Part 11 (electronic record keeping); for the EU, it will generate German, French, and Spanish versions of compliance reports; for Southeast Asia, it will adapt to tropical high-temperature environments (with a cabinet cooling system). This helps manufacturers enter global markets quickly.
- Green and Low-Carbon Design
With the focus on carbon neutrality, BIAMs will adopt energy-saving components (e.g., servo motors with 96% energy efficiency) and recyclable materials (e.g., biodegradable silicone suction nozzles). Some manufacturers are developing “low-waste systems” that collect and reuse unqualified components (after re-sterilization), reducing material waste by 30%.
Conclusion: More Than an Assembly Machine—A “Cornerstone” of Public Health Safety
The Biological Indicator Assembly Machine is not just a production tool; it is a critical link in the global sterilization safety chain. Every BI assembled by the machine is a “silent inspector”—it verifies that surgical instruments are sterile, drugs are safe, and food is free from microbial contamination, ultimately protecting patients, consumers, and public health.
As China’s BI industry accelerates its localization and globalization, high-performance BIAMs will not only reduce reliance on imported equipment (which previously accounted for 70% of the market) but also help domestic BI brands meet international standards. In the future, with further technological upgrades, BIAMs will continue to be the “invisible guardians” of sterilization safety, contributing to a healthier global public health system.