Table of Contents
ToggleBreakthrough in Food and Pharmaceutical Industry: Flexible Automation Solves “Multi-Specification, Strict Compliance” Production Challenges
Scene Spotlight:
In the solid dosage workshop of a pharmaceutical enterprise, Production Supervisor Sister Chen is in a panic staring at the upcoming rectification notice — the delay in switching packaging specifications from 100 tablets/bottle to 500 tablets/bottle within 4 hours has postponed the new product launch; more seriously, incomplete cleaning after changeover last week led to residue from previous products detected in a batch of capsules, triggering a FDA warning letter and direct economic losses exceeding 20 million yuan. In the food and pharmaceutical industry, “rapid multi-specification switching” and “zero-contamination compliant production” act like double-edged swords, cutting into enterprises’ profits and reputations.
I. Three “Flexibility Shackles” in Food and Pharmaceutical Industry
1. Explosive Growth of Specifications, Staggering Changeover Costs
Market-driven Pressures:
- Personalized demands explode in the food industry (e.g., sugar-free/low-sugar/gluten-free niche products), with SKUs in a dairy factory increasing from 50 to 300, and traditional production line changeover frequency surging from 2 to 15 times daily;
- The pharmaceutical industry requires multi-specification packaging (e.g., 2ml/5ml/10ml injections) and multi-dose specifications (e.g., 10 tablets/board, 30 tablets/board). Each changeover requires equipment cleaning and metering pump calibration, taking 2-4 hours and causing 15% annual capacity loss.
2. Strict Compliance Barriers, One Misstep Leads to Total Loss
GMP Iron Rules:
- Equipment surface roughness must be ≤0.8μm (to avoid material residue). A food factory incurred 1.2 million yuan in rework costs due to failing cleaning validation with ordinary stainless steel fixtures;
- Batch traceability requires recording over 30 dimensions of data (e.g., mixing time, sterilization temperature, operator). Traditional manual entry has a 5% error rate, with a pharmaceutical company fined 5 million yuan for incomplete traceability data.
3. High Incidence of Quality Risks, Making Flexible Production a Tightrope Walk
Cross-Contamination Hazards:
- In tablet production, residue of raw material powders from different varieties can cause ingredient overstandardization. Incomplete changeover cleaning in a factory triggered 2,000 consumer allergy complaints, shrinking brand value by 30%;
Fatal Metering Precision:
- Pharmaceutical preparation filling precision requires ±0.5% (e.g., insulin injection dosage deviation over 1% renders it ineffective). Traditional manual calibration takes 15 minutes per instance, with a missed detection rate of 0.2%.
II. “Clean Flexibility” Solutions for Flexible Automation Breakthrough
1. Quick Changeover + Aseptic Cleaning: Creating “Zero-Residue” Production Units
(1) Modular Clean Quick-Change System
- Hardware Design:
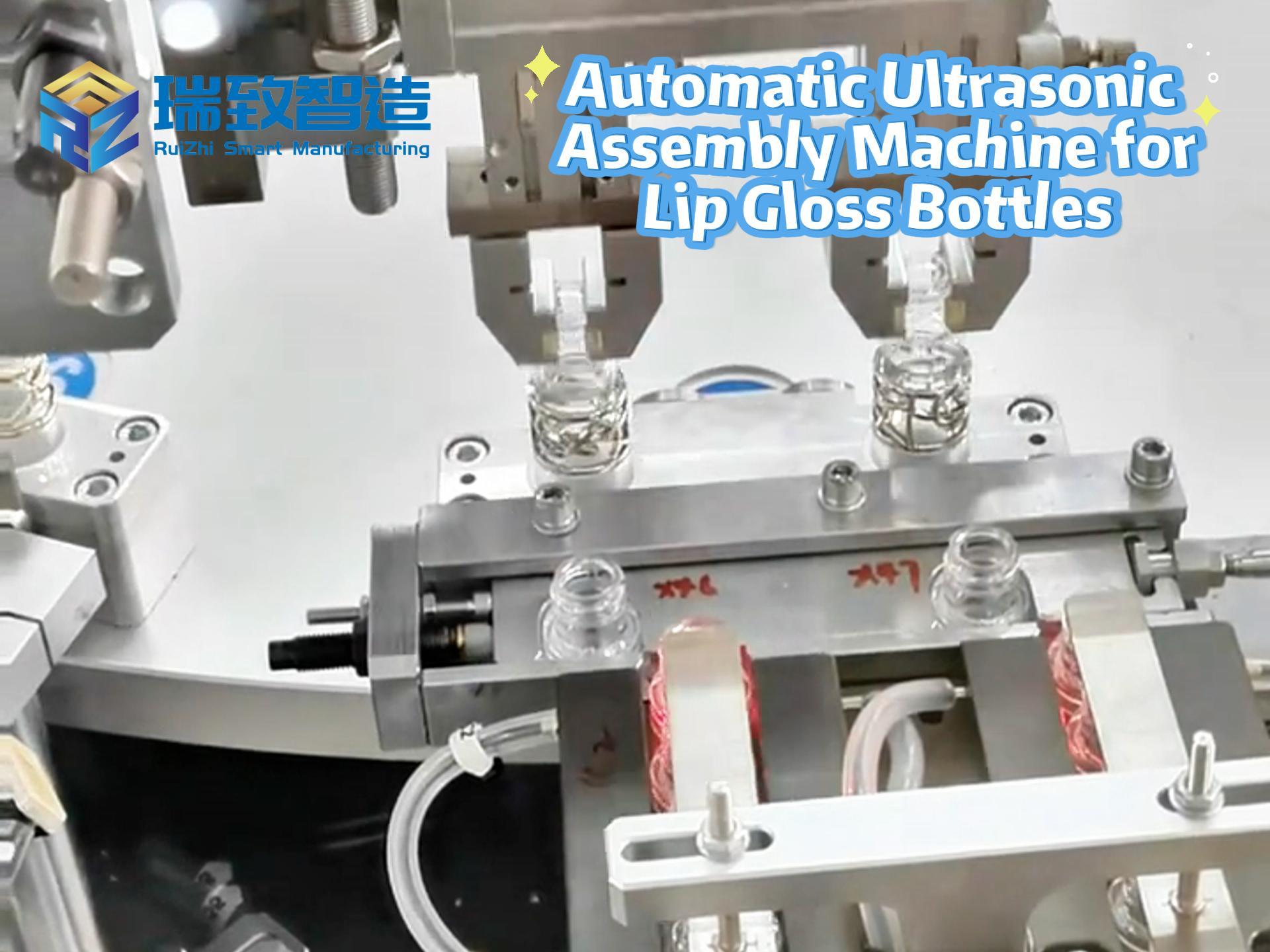
- Adopt electromagnetic induction quick-change fixtures (surface electrolytically polished to roughness ≤0.4μm), paired with camera automatic alignment, reducing changeover time from 120 minutes to 15 minutes;
- Case: After introducing the quick-change system, an oral liquid pharmaceutical enterprise can produce 8 specifications daily, shortening changeover cleaning time by 75% and reducing new product launch cycles from 30 days to 10 days.
- Cleaning Validation:
- Integrate CIP (Clean-In-Place) + SIP (Sterilize-In-Place) modules, automatically flushing pipelines after changeover (85℃ water, 3bar pressure), and detecting residue via infrared sensors, reducing cleaning validation time from 60 minutes to 10 minutes.
(2) Intelligent Visual Alignment Technology
- Pharmaceutical Scenarios:
- Injection filling machines equipped with laser ranging + 3D vision systems automatically identify ampoule positions, achieving filling precision of ±0.3%. A factory obtained a $5 million order by passing WHO pre-certification;
- Food Scenarios:
- Chocolate packaging lines use vision recognition systems to detect nut distribution, ensuring nut count error ≤1 per chocolate piece, reducing customer complaint rates by 90%.
2. Full-chain Digital Traceability: Shifting Compliance from “Post-event Reconciliation” to “Real-time Accounting”
(1) Blockchain + IoT Traceability Network
- Data Collection:
- Each production batch generates an independent blockchain ledger, real-time recording data such as raw material weighing (0.1g precision), mixing time (1-second precision), and sterilization parameters (temperature ±0.5℃), which are tamper-proof and searchable within seconds;
- After application, a milk powder factory reduced regulatory inspection response time from 3 days to 2 hours, successfully passing BRC A-level certification.
(2) AI Quality Early Warning
- Edge computing modules analyze metal detector data in real time, triggering shutdown within 0.2 seconds upon detecting foreign objects and automatically isolating the preceding and following 100 products. A biscuit factory reduced foreign object complaints from 0.5 times/10,000 pieces to 0.05 times/10,000 pieces.
3. Flexible Packaging: From “Single Specification” to “Endless Variety”
(1) Adaptive Packaging Units
- Hardware:
- Adjustable forming molds (e.g., pharmaceutical blister packaging machines automatically adjust blister sizes via servo motors, supporting 10-50mm diameter ranges);
- Food industry application: A snack factory’s flexible packaging line can handle 50g sachets, 200g boxes, and 500g cans simultaneously, requiring no mechanical part replacement for changeover—only program parameter adjustment.
(2) Intelligent Labeling Systems
- Vision positioning + dynamic printing technology ensure label position deviation ≤1mm (meeting pharmaceutical regulatory requirements for clear batch information). A pharmaceutical enterprise increased labeling efficiency by 40% and reduced label re-sticking rates from 8% to 1%.
III. “Clean Implementation Path” for Food and Pharmaceutical Flexibility Transformation
1. Compliance Priority: Mapping the “Clean Flexibility” Transformation Roadmap
(1) Certification-oriented Equipment Selection
- Must pass certifications such as FDA 21 CFR Part 11 (electronic record compliance) and EU GMP Annex 1 (aseptic production requirements);
- Case: A medical device factory delayed entering the European market by 6 months due to equipment failing CE MDR certification, a painful lesson.
(2) Cleaning Validation First
- Complete cleaning validation plan design before transformation (e.g., identifying 最难清洁部位 (most difficult-to-clean areas) and setting residue limits). A food factory collaborated with a third-party testing agency 3 months in advance to ensure one-time audit pass after transformation.
2. Stage-by-stage Transformation: From “Clean Workstations” to “Intelligent Production Lines”
(1) Workstation-level Transformation (3-6 months)
- Prioritize bottleneck workstations:
- Pharmaceuticals: Filling/labeling workstations (introduce servo metering pumps + visual labeling machines, improving dosage precision to ±0.2% and labeling efficiency by 50%);
- Food: Sorting/packaging workstations (deploy collaborative robots + food-grade grippers, adapting to sorting irregular materials like biscuits and candies, reducing breakage rates from 3% to 0.5%).
(2) Production Line-level Integration (6-12 months)
- Build a clean data middle platform:
- Unify equipment communication protocols (e.g., use OPC UA secure communication compliant with FDA requirements), enabling automatic data collection from raw material weighing to finished product warehousing;
- After integration, a health product factory reduced batch traceability time from 4 hours to 10 minutes and cut compliance costs by 60%.
3. Personnel Capability Upgrade: Cultivating “Clean Smart Technicians”
(1) Dual Certification System:
- GMP compliance certification (mastering equipment cleaning validation processes and abnormal data handling);
- Flexible operation certification (completing clean fixture switching within 10 minutes and CIP/SIP operations within 20 minutes), with certified employees in a pharmaceutical enterprise receiving 40% salary increases;
(2) Simulation Training System:
- Build 1:1 clean workshop simulation environments for practicing changeover cleaning and abnormal shutdown handling, increasing new employee compliance operation pass rates from 60% to 95%.
IV. Benchmark Case: The Counterattack from “Compliance Nightmare” to “Dual Champion of Flexibility and Compliance”
Case: A Chinese Factory of a Multinational Pharmaceutical Enterprise (Annual Output Value >2 Billion Yuan)
Pain Points:
- Multi-specification tablet production changeover took 4 hours, causing annual capacity loss of 50 million tablets;
- Repeated warnings from the EU 药监 (drug regulatory) authority due to failed cleaning validation, facing product ban risks.
Transformation Path:
- Hardware Upgrade:
- Introduce German customized flexible tablet presses supporting 10-30mm diameter tablet switching, compressing changeover time to 20 minutes, with built-in automatic cleaning brushes (99.9% residual powder removal efficiency);
- Deploy 30 sets of pharmaceutical-grade visual inspection systems, real-time scanning for tablet surface defects (e.g., chipping, weight deviation), reducing missed detection rates from 0.1% to 0.01%.
- Software Reengineering:
- Develop a “compliance flexible scheduling system” to automatically match optimal production routes (prioritizing high-compliance-risk export orders), reducing export order delivery cycles from 15 days to 5 days;
- Blockchain traceability systems cover over 50 processes from raw materials to finished products, reducing FDA unannounced inspection response time from 3 days to 30 minutes.
- Effectiveness Data:
- Multi-specification changeover efficiency increased by 80%, annual capacity increased by 30 million tablets, new revenue of 120 million yuan;
- 100% one-time cleaning validation pass rate, successfully passing dual certification from the EU and FDA, obtaining 3 innovative drug production approvals;
- Quality costs decreased by 40%, ROI reached 180%, payback in 15 months.
V. “Iron Rules for Pitfall Avoidance” in Food and Pharmaceutical Flexibility Transformation
1. Compliance Details Determine Success or Failure
- A food factory ignored equipment material certification and used ordinary lubricating oil (containing silicon), causing contamination of the biscuit production line and destruction of the entire batch, resulting in 8 million yuan in losses;
- Must-check items: Equipment materials must comply with standards such as FDA 21 CFR 177.2600 (food contact materials) and ISO 10993 (medical device biocompatibility).
2. Don’t Let “Flexibility” Sacrifice “Cleanliness”
- A pharmaceutical enterprise simplified cleaning steps in pursuit of changeover speed, leading to excessive residue and being blacklisted by WHO, restricting global market access;
- Principle: Flexible transformation must prioritize compliance, with cleaning validation taking precedence over efficiency improvement.
3. Calculate “Hidden Compliance Costs” Clearly
- Hidden costs such as manual records and repeated cleaning in traditional production lines account for 12% of production costs. After transformation, a factory reduced hidden costs by 8% through automated traceability and rapid cleaning, equivalent to annual savings of 16 million yuan.
Act Now: Food and Pharmaceutical Flexibility Transformation “Compliance Starter Pack”
- Scan to receive the Food and Pharmaceutical Compliance Flexibility Transformation Checklist
- Includes 20 equipment material detection items, 15 cleaning validation key points, and 10 sets of GMP record templates;
- Schedule a “Clean Flexibility Diagnosis Service”
- Senior GMP consultants will visit to assess production line compliance loopholes and provide a Clean Flexibility Transformation Feasibility Report(including certification pass roadmaps);
- Join the “Food and Pharmaceutical Intelligent Compliance Alliance”
- Share FDA/EU certification experiences and cleaning validation best practices, and access the Aseptic Production Flexibility White Paper.
In the food and pharmaceutical industry, flexible automation is not an “efficiency tool” but a “survival necessity.” As consumer demands for personalized food surge and pharmaceutical regulatory compliance nets tighten, only enterprises that deeply integrate “flexible production” with “clean compliance” can walk steadily on the tightrope of “rapid multi-specification switching” and “zero-risk quality control.” The next issue, Breakthrough in Logistics and Warehousing Industry: How Flexible Automation Solves “Multi-SKU, Fast-Turnover” Sorting Challenges, will focus on warehousing and logistics, analyzing how automation addresses the extreme challenges of e-commerce logistics. Stay tuned!
(For more flexible materials on the food and pharmaceutical industry or to discuss specific transformation details, feel free to communicate at any time!)
#machine vision singapore #machine vision automation #automatic seal assembly